iminodiacetic acid

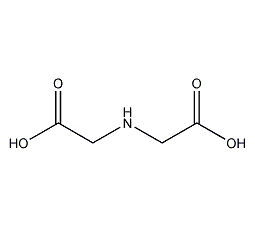
Structural formula
| Business number | 03VB |
|---|---|
| Molecular formula | C4H7NO4 |
| Molecular weight | 133.10 |
| label |
None yet |
Numbering system
CAS number:142-73-4
MDL number:MFCD00004280
EINECS number:205-555-4
RTECS number:AI2975000
BRN number:878499
PubChem number:24880393
Physical property data
1. Physical property data
1. Appearance: white powder
2. Melting point (ºC): 243 ºC
3. Relative density (ºC, 25/4): 1.56
4 . Solubility: Soluble in Water, insoluble in alcohol, acetone and ether.
Toxicological data
2. Toxicology data:
1. Acute toxicity:
Mouse abdominal LD50: 250 mg/kg
Ecological data
3. Ecological data:
Other harmful effects: This substance may be harmful to the environment, and special attention should be paid to water bodies.
Molecular structure data
5. Molecular property data:
1. Molar refractive index: 27.30
2. Molar volume (cm3/mol): 92.6
3. Isotonic specific volume (90.2K): 262.8
4. Surface tension (dyne/cm): 64.8
5. Polarizability (10-24cm3): 10.82
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): -3.3
2. Number of hydrogen bond donors: 3
3. Number of hydrogen bond acceptors: 5
4. Number of rotatable chemical bonds: 4
5. Number of tautomers: none
6. Topological molecule polar surface area 86.6
7. Number of heavy atoms: 9
8. Surface charge: 0
9. Complexity: 108
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Stable under normal temperature and pressure.
Incompatible materials: oxidizing agent
2.Form salts with acids and bases, and also form chelates with various metals
Storage method
1. Store in a dry and sealed container at room temperature.
2.Do not store or transport together with acids, alkalis, etc.
Synthesis method
1. Sodium chloroacetate method: Sodium chloroacetate is prepared from chloroacetic acid, and then reacts with hydrazine hydrate to generate hydrazine diacetic acid, and finally iminoacetic acid is prepared under the action of sodium nitrite. 
2. Chloroacetic acid and aminoacetic acid method
3. Glycine and glycolonitrile method

Purpose
1. Iminodiacetic acid is an intermediate of the herbicide glyphosate.
2. Used in pesticides, rubber and aminocarboxylic complexes, and widely used as raw materials for glyphosate.
3. Used as complexing agent and also used in organic synthesis .
4. Used in the synthesis of glyphosate. It is also used as a synthetic raw material for amino acid chelating resin. It is also an important raw material and intermediate in the rubber and electroplating industries. It is also used as a surfactant and complex. Mixture intermediate.
5.Iminodiacetic acid is used as a complexing agent for electroplating or electroless plating.
