Lauryl methacrylate
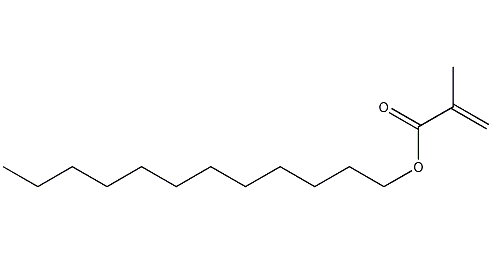

Structural formula
| Business number | 03VK |
|---|---|
| Molecular formula | C16H30O2 |
| Molecular weight | 254.41 |
| label |
Lauryl methacrylate, Lauryl methacrylate, 2-Methyl-2-propenoate dodecyl ester, Lauryl methacrylate, Lauryl methacrylate, Lauryl methacrylate, Dodecyl 2-methyl-2-propenoate, 2-Methyl-2-propenoic acid dodecyl ester |
Numbering system
CAS number:142-90-5
MDL number:MFCD00008972
EINECS number:205-570-6
RTECS number:OZ4300000
BRN number:1708160
PubChem number:24857542
Physical property data
1. Physical property data
1. Properties: colorless liquid
2. Density (g/mL, 25/4℃): 0.868
3. Solubility: soluble in most organic solvents.
4. Melting point (ºC): -7–13 ºC
5. Boiling point (ºC, normal pressure): 1424ºC
6. Refractive index: 1.445
7. Relative density (20℃, 4℃): 0.8735
8. Refractive index at room temperature (n20): 1.4452
9. Normal temperature refractive index (n25): 1.444
Toxicological data
2. Toxicology data:
1. Acute toxicity: rat abdominal LD50: 12 mg/kg;
Mouse abdominal LD50: 25 mg/kg.
Ecological data
3. Ecological data:
Other harmful effects: This substance may be harmful to the environment, and special attention should be paid to water bodies.
Molecular structure data
5. Molecular property data:
1. Molar refractive index: 77.51
2. Molar volume (cm3/mol): 291.0
3 , Isotonic specific volume (90.2K): 678.6
4. Surface tension (dyne/cm): 29.5
5. Polarizability (10-24 cm3): 30.73
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 13
5. Number of tautomers: none
6. Topological molecule polar surface area 26.3
7. Number of heavy atoms: 18
8. Surface charge: 0
9. Complexity: 221
10. Number of isotope atoms: 0
11. Determined number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of stereocenters of chemical bonds: 0
14. Uncertain number of stereocenters of chemical bonds: 0
15. Number of covalent bond units: 1
Properties and stability
Stable under normal temperature and pressure.
Incompatible materials: strong oxidizing agents.
Storage method
Save at 0-6°C.
Synthesis method
1. Preparation method:
In a reaction flask equipped with a Webster fractionating column, add 120.2g (1.2mol) of methyl methacrylate (2) and 74.6g (0.4mol) of lauryl alcohol ), 5.5g of hydroquinone, 2g of p-toluenesulfonic acid (or 0.5mL of concentrated sulfuric acid), and inert gas replacement 3 times. Heat to reflux under magnetic stirring for 6 to 10 hours, during which the resulting azeotrope of methanol and methyl methacrylate is continuously evaporated, with a boiling point of 55 to 65°C, until there is no distillate. The water pump decompresses and evaporates the remaining small amount of methyl methacrylate. Cool, wash with saturated sodium bicarbonate solution and water in sequence, and dry over anhydrous sodium sulfate. Add a little phenothiazine, fractionate under reduced pressure, and collect the fractions at 140-145°C/532Pa to obtain 280g of compound (1) with a yield of 92%. [1]
Purpose
Used in the manufacture of lubricating oil additives, deodorants, paper processing agents, adhesives, leather agents and internal plasticizers, etc.
