Nonyl acetate
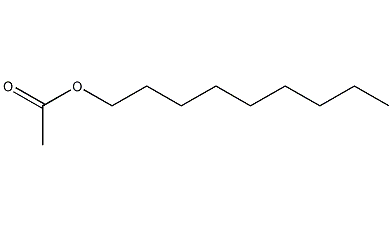

Structural formula
| Business number | 03VT |
|---|---|
| Molecular formula | C11H22O2 |
| Molecular weight | 186.29 |
| label |
n-nonyl acetate, N-Nonyl acetate, 1-Acetoxynonane |
Numbering system
CAS number:143-13-5
MDL number:MFCD00027340
EINECS number:205-585-8
RTECS number:AJ1382500
BRN number:None
PubChem number:24901329
Physical property data
1. Physical property data
1. Properties: colorless liquid
2. Density (g/mL, 20℃): 0.86
3. Flash point (ºC): 67.2 ºC
4. Boiling point (ºC, normal pressure): 210ºC
5. Refractive index: 1.424
6. Solubility : Insoluble in water, soluble in alcohol and ether.
7. Melting point (ºC): -26
8. Relative density (20℃, 4℃): 0.8655
9. Relative density (25℃ , 4℃): 0.8612
10. Refractive index at room temperature (n20): 1.4239
11. Refractive index at room temperature (n25): 1.4225
Toxicological data
2. Toxicological data:
1. Acute toxicity: Rat oral LD50: >5 mg/kg;
Rat dermal LD50: >5 mg/kg kg.
Ecological data
3. Ecological data:
Usually it is not harmful to water. Do not discharge materials into the surrounding environment without government permission.
Molecular structure data
5. Molecular property data:
1. Molar refractive index: 54.78
2. Molar volume (cm3/mol): 213.5
3. Isotonic specific volume (90.2K): 494.5
4. Surface tension (dyne/cm): 28.7
5. Polarizability (10-24cm3): 21.71
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 9
5. Number of tautomers: none
6. Topological molecule polar surface area 26.3
7. Number of heavy atoms: 13
8. Surface charge: 0
9. Complexity: 121
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
Stable under normal temperature and pressure.
Incompatible materials: strong oxidants, strong acids, and strong bases.
Storage method
Store sealed in a dry and cool place.
Synthesis method
It is produced by direct esterification of nonanol and acetic acid.
Purpose
1.GB 2760-96 stipulates that edible spices are allowed to be used. Mainly used to prepare apricot, peach, lemon and other citrus flavors.
2. Can be used in small amounts in many fragrances, such as orange blossom, rose, gardenia, lavender, violet, parsnip, iris, citrus and oriental. It can harmonize well in floral and non-floral scents, and can enhance the violet aroma of ionone and the fresh fragrance of Gepon. It can also be used in edible apricots, peaches, lemons and other citrus and fruity flavors.
