Hexadecylamine
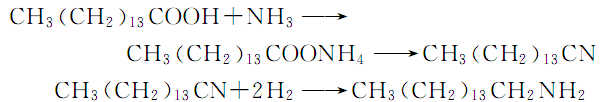
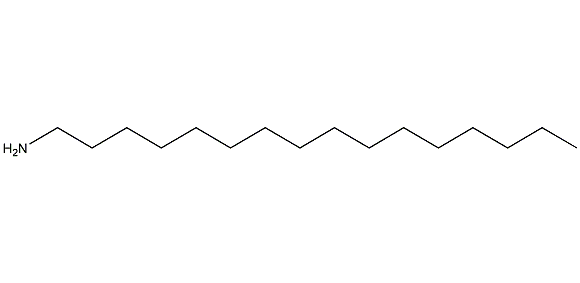
Structural formula
| Business number | 03W1 |
|---|---|
| Molecular formula | C16H35N |
| Molecular weight | 241.46 |
| label |
1-Hexadecylamine, stearylamine, Cetylamine, Hexadecylamine, cetamine, 1-Aminohexadecane, 1-Aminohexadecane, 1-Hexadecylamine, Cetylamine, Organic corrosion inhibitor materials |
Numbering system
CAS number:143-27-1
MDL number:MFCD00008158
EINECS number:205-596-8
RTECS number:ML9100000
BRN number:1634065
PubChem number:24868173
Physical property data
1. Physical property data;
1. Properties: white flaky crystalline powder
2. Relative density (20/4℃): 0.8129
3. Refractive index: 1.4496
4. Flash point (℃): 140
5. Melting point (℃): 46.77
6. Boiling point (ºC) : 332.5
7. Vapor pressure (kPa, 25ºC): 0.0003
8. Heat of combustion (KJ/mol): 56.4
9. Oil and water (porous Log value of the partition coefficient (alcohol/water):
10. Solubility: Soluble in ethanol, ether, acetone, benzene and chloroform, insoluble in water.
Toxicological data
2. Toxicological data:
1. Acute toxicity: mouse abdominal LD50: 200 mg/kg.
2. Reproductive toxicity
Small Mouse oral TDLo: 40 mg/kg;
Mouse intraperitoneal TDLo: 10 mg/kg.
Ecological data
3. Ecological data:
Other harmful effects: This substance may be harmful to the environment, and special attention should be paid to water bodies.
Molecular structure data
5. Molecular property data:
1. Molar refractive index: 79.71
2. Molar volume (cm3/mol): 296.3
3 , Isotonic specific volume (90.2K): 697.7
4. Surface tension (dyne/cm): 30.7
5. Polarizability (10-24 cm3): 31.59
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 14
5. Number of tautomers: none
6. Topological molecule polar surface area 26
7. Number of heavy atoms: 17
8. Surface charge: 0
9. Complexity: 123
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Stable under normal temperature and pressure.
2. Incompatible materials:Strong oxidants
Storage method
Store sealed in a dry and cool place.
Synthesis method
In industrial production, hexadeconitrile can be prepared from palmitic acid and ammonia, and then catalytic hydrogenation under pressure can be produced. Hydrogenation uses Co or Ni as catalyst, the temperature is 120~180℃, and the pressure is 2~10mPa. The reaction formula is:
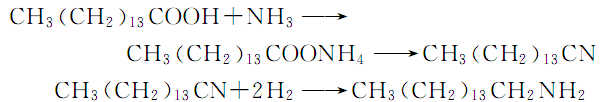
Obtained from preserved palm fruit through reduction. Dissolve 3 parts of palm fruit in 30 parts of absolute ethanol, and slowly add 4 parts of metallic sodium for reduction. The reaction releases heat and makes the reactants in a liquid state. When the added metallic sodium can only be partially dissolved, it can be heated to 60°C and gradually raised to 120°C so that the metallic sodium does not completely disappear. After the reaction is complete, pour it into water while it is hot, add a slight excess of hydrochloric acid, filter out the resulting hexadecylamine hydrochloride crystals, wash with a small amount of dilute hydrochloric acid and press dry. Alkalize the hydrochloric acid with 20% sodium oxychloride and distill under reduced pressure to obtain hexadecylamine.
Purpose
1. Used to prepare resins, pesticides and high-grade detergents.
2. Used as fiber additives, fertilizer anti-caking agents, flotation agents, etc.
3.Used as a corrosion inhibitor for water systems such as low-pressure boiler water supply systems with high alkalinity and circulating cooling water systems, condensate water systems and circulating Corrosion inhibitor for cooling water.
4.Used as fabric softener, detergent, mineral flotation agent, anti-caking agent, bactericidal disinfectant, and coating It can also be used as dispersant for pigments, antistatic agent for plastics, etc., and can also be used to prepare resin. It can form an extremely dense monomolecular layer adsorption film on the metal surface, and has a good anti-corrosion effect on iron and copper.
