Erythritol
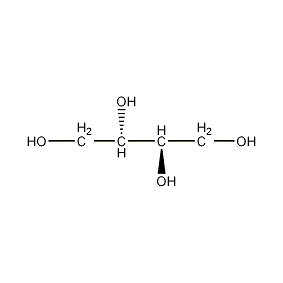

Structural formula
| Business number | 03XR |
|---|---|
| Molecular formula | C4H10O4 |
| Molecular weight | 122.12 |
| label |
(R,S)-1,2,3,4-butanetetraol, Erythritol, erythritol, erythritol, protoalcohol, 1,2,3,4-Butanetetrol, Erythrite, Diluents, humectants, flavor enhancers, texture improvers and molding aids for high-intensity sweeteners, alcohol solvents |
Numbering system
CAS number:149-32-6
MDL number:MFCD00004710
EINECS number:205-737-3
RTECS number:KF2000000
BRN number:1719753
PubChem number:24894646
Physical property data
1. Properties: white crystal
2. Melting point (℃): 120~123
3. Boiling point (℃): 329~331
4. Relative density (g/mL, air = 1): 1.451
5. Flash point: 329-331℃
6. Solubility: Easily soluble in water, soluble in water In pyridine, slightly soluble in alcohol, almost insoluble in ether.
7. Boiling point (ºC): 330.5
8. Relative density (20℃, 4℃): 1.451
9. Gas phase standard heat of combustion (enthalpy )(kJ·mol-1): -2227.9
10. The gas phase standard claims heat (enthalpy) (kJ·mol-1): – 775.2
11. Liquid phase standard combustion heat (enthalpy) (kJ·mol-1): -2116.1
12. Liquid phase standard claimed heat ( Enthalpy) (kJ·mol-1): -887.0
13. Crystal phase standard combustion heat (enthalpy) (kJ·mol-1 ): -2092.8
14. Crystal phase standard claims heat (enthalpy) (kJ·mol-1): -910.4
Toxicological data
Acute toxicity: mouse abdominal LD50: 7 gm/kg
Ecological data
Other harmful effects: This substance may be harmful to the environment, and special attention should be paid to water bodies.
Molecular structure data
1. Molar refractive index: 26.63
2. Molar volume (cm3/mol): 85.3
3. Isotonic specific volume (90.2K): 252.9
4. Surface tension (dyne/cm): 77.2
5. Polarizability (10-24cm3): 10.55
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): -2.3
2. Number of hydrogen bond donors: 4
3. Number of hydrogen bond acceptors: 4
p>
4. Number of rotatable chemical bonds: 3
5. Topological molecular polar surface area (TPSA): 80.9
6. Number of heavy atoms: 8
7. Surface charge: 0
8. Complexity: 48
9. Number of isotope atoms: 0
10. Determine the atomic stereocenter Number: 2
11. Number of uncertain atomic stereocenters: 0
12. Confirmed��Number of stereocenters of chemical bonds: 0
13. Number of stereocenters of uncertain chemical bonds: 0
14. Number of covalent bond units: 1
Properties and stability
1. Stable under normal temperature and pressure. Incompatible materials: strong oxidants
2. Exist in mainstream flue gas.
Storage method
Plastic bag coated with kraft paper bag, sealed packaging, stored in a cool and dry place. Do not store together with toxic and polluted items.
Synthesis method
1. Erythritol can be extracted from seaweed, moss and certain grasses. It can be synthesized artificially by reacting butylene glycol and hydrogen peroxide. Among them, butene diol is produced by first making 2-butyne-1,4-diol from acetylene and formaldehyde, then mixing its aqueous solution with Raney nickel, adding inhibitor ammonia, and hydrogenating it at about 0.5MPa. .
2. Made from wheat, corn and other starches through safe and appropriate food-grade hypertonic yeasts such as Moniliella pllinis, Candida lipolytica or hyphomycetes ( After enzymatic fermentation of Tricho sporonoides megachilensis) at high concentration (>450g/L), the fermentation mash is heated and sterilized, filtered and then purified by ion exchange resin, activated carbon and ultrafiltration, crystallized, washed and dried. The general yield is about 50%.
3. After starch milk is enzymatically decomposed into glucose, it is fermented by hypertonic yeast at high concentrations and then concentrated, crystallized, separated and dried.
Purpose
It can be used as diluent, humectant, flavor enhancer, texture improver and molding aid for low-calorie sweeteners and high-intensity sweeteners. It can also be used in chocolate, baked products, soft drinks, solid drinks, candies, etc. It is especially suitable for the production of food that avoids moisture.
