1-nitronaphthalene
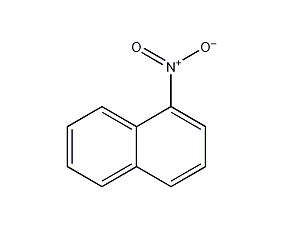

Structural formula
| Business number | 01WW |
|---|---|
| Molecular formula | C10H7NO2 |
| Molecular weight | 173 |
| label |
None yet |
Numbering system
CAS number:86-57-7
MDL number:MFCD00003913
EINECS number:201-684-5
RTECS number:QJ9720000
BRN number:1867714
PubChem number:24886577
Physical property data
1. Character: yellow needle crystal[1]
2. Melting point (℃): 58.8~61[2]
3. Boiling point (℃): 304[3]
4. Relative density (water=1): 1.33[4]
5. Relative vapor density (air=1): 5.96[5]
6. Octanol/water partition coefficient: 3.19 [6]
7. Flash point (℃): 164 (CC) [7]
8. Solubility: insoluble in Water, soluble in ethanol, ether, chloroform, carbon disulfide, easily soluble in benzene and pyridine. [8]
Toxicological data
1. Acute toxicity[9]
LD50: 150mg/kg (rat oral); 86mg/kg (rat intraperitoneal )
2. Irritation No information available
3. Mutagenicity[10] Microbial mutagenicity: Salmonella typhimurium 100μg/dish. DNA inhibition: human HeLa cells 260 μmol/L. Sister chromatid exchange: hamster lung 20mg/L. Mammalian somatic mutation: hamster lung 20mg/L.
4. Carcinogenicity [11] IARC Carcinogenicity Comment: G3, insufficient evidence of carcinogenicity to humans and animals.
Ecological data
1. Ecotoxicity[12] LC50: 9mg/L (96h) (fathead minnow)
2. Biological Degradability No data available
3. Non-biodegradability No data available
Molecular structure data
1. Molar refractive index: 50.64
2. Molar volume (cm3/mol): 135.3
3. Isotonic specific volume (90.2K ): 366.5
4. Surface tension (dyne/cm): 53.7
5. Polarizability (10-24cm3):20.07
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: none
6. Topological molecule polar surface area 45.8
7. Number of heavy atoms: 13
8. Surface charge: 0
9. Complexity: 200
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Stability[13] Stable
2. Incompatible substances[14] Strong oxidizing agent, strong reducing agent
3. Conditions to avoid contact[15] Heating
4. Polymerization hazard[16] No polymerization
5. Decomposition products [17] Nitrogen oxides
Storage method
Storage Precautions[18] Store in a cool, ventilated warehouse. The storage temperature should not exceed 35℃. Keep away from fire and heat sources. The packaging is sealed. They should be stored separately from oxidants and reducing agents, and avoid mixed storage. Use explosion-proof lighting and ventilation facilities. It is prohibited to use mechanical equipment and tools that are prone to sparks. Suitable materials should be available in the storage area to contain spills.
Synthesis method
Obtained from the nitration of naphthalene. In actual industrial production, part of the mixed acid required for the reaction can be reused. Add 600kg of the waste acid obtained from the previous batch of nitration into the glass-lined reaction pot, add 300kg of 98% sulfuric acid and 793kg of 100% nitric acid under cooling, and stir to prepare a mixed acid for use. Add 800kg of waste acid from the previous nitration to the reaction pot, add 1550kg of refined naphthalene and the mixed acid prepared above, and add the refined naphthalene and mixed acid in three increments, and control the temperature at 50-55°C. After the addition is completed, raise the temperature to 65°C and keep warm for 3 hours. At the end of sampling inspection, when the freezing point is ≥51°C, the nitrification waste acid is separated. Wash the reactant with hot water until neutral to obtain 1-nitronaphthalene.
Purpose
Used in the manufacture of dyes and used in the petroleum industry to remove fluorescence. [19]
