N,N-diethyl o-nitroaniline
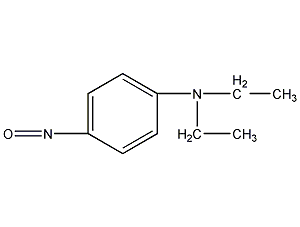

Structural formula
| Business number | 03BV |
|---|---|
| Molecular formula | C10H14N2O |
| Molecular weight | 178.24 |
| label |
4-Nitroso-N,N-diethylaniline, p-Nitroso-N,N-diethylaniline, 4-Nitroso-N,N-diethylaniline, 4-Diethylaminonitrosobenzene, Diethyl-p-nitrosoaniline, aromatic compounds |
Numbering system
CAS number:120-22-9
MDL number:MFCD00002064
EINECS number:204-379-5
RTECS number:BX3610000
BRN number:None
PubChem number:24848179
Physical property data
1. Characteristics: Green powder.
2. Density (g/mL,15℃): 1.24
3. Relative vapor density ( g/mL,Air =1): Undetermined
4. Melting point (ºC):82~ 84
5. Boiling point (ºC,normal pressure): Undetermined
6. Boiling point (ºC,kPa): Undetermined
7. Refractive index: Undetermined
8. Flashpoint (ºC): Undetermined
9. Specific rotation (º): Undetermined
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (mmHg,25ºC): Undetermined
12. Saturated vapor pressure (kPa, ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Oil and water (octanol/Log value of the partition coefficient for water: undetermined
17. 3. isotonic ratio (90.2K): 430.1
4. Surface Tension (dyne/cm):35.6
5. Polarizability(10-24cm3):21.23
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 2.1
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 3
4. Number of rotatable chemical bonds: 3
5. Number of tautomers: none
6. Topological molecule polar surface area 32.7
7. Number of heavy atoms: 13
8. Surface charge: 0
9. Complexity: 149
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
Avoid contact with oxides and acetic acid.
Storage method
Use water as a stabilizer during storage. Store in a cool, ventilated warehouse. Keep away from fire and heat sources. Protect from direct sunlight. Keep container sealed and strictly prohibited from contact with air. Should be used with oxidants, acetic acid, food
Chemicals should be stored separately and avoid mixed storage. Use explosion-proof lighting and ventilation facilities. It is prohibited to use mechanical equipment and tools that are prone to sparks. Suitable materials should be available in the storage area to contain spills.
Synthesis method
Replace N,N-diethylaniline, The mixture of concentrated hydrochloric acid and ethanol is cooled to 0℃The following, mix with isoamyl nitrite and stir for reaction2-3h. The reaction solution was extracted with diethyl ether to remove isoamyl alcohol. For remaining liquid
Neutralize excess sodium carbonate solution until it becomes alkaline, and extract with ether. The ether extract is salted with concentrated sulfuric acid in absolute ethanol and filtered to obtain p-nitroso-N,N-diethylaniline sulfate, which is treated with carbonic acid
Sodium neutralizes and precipitates free p-nitroso group-N, N-Diethylaniline.
Purpose
For organic synthesis.
�The solution was neutralized until it became alkaline and extracted with diethyl ether. The ether extract is salted with concentrated sulfuric acid in absolute ethanol and filtered to obtain p-nitroso-N,N-diethylaniline sulfate, which is treated with carbonic acid
Sodium neutralizes and precipitates free p-nitroso group-N, N-Diethylaniline.
Purpose
For organic synthesis.
