N-O-anilinobenzoic acid
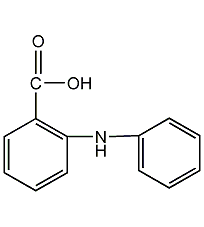

Structural formula
| Business number | 023F |
|---|---|
| Molecular formula | C13H11NO2 |
| Molecular weight | 213.23 |
| label |
N-phenyl anthranilic acid, 2-(phenylamino)benzoic acid, 2-(Phenylamino)benzoic acid, Diphenylamine-2-carboxylic acid, DPC, 2-(C6H5NH)C6H4CO2H |
Numbering system
CAS number:91-40-7
MDL number:MFCD00002421
EINECS number:202-066-8
RTECS number:CB3730000
BRN number:1456607
PubChem number:24887261
Physical property data
1. Characteristics: white flaky crystals.
2. Density (g/mL,25/4℃): Undetermined
3. Relative vapor density (g/mL,AIR=1): Undetermined
4. Melting point (ºC): 183-184
5. Boiling point (ºC,Normal pressure): Undetermined
6. Boiling point (ºC,5.2kPa): Undetermined
7. Refractive Index: Undetermined
8. Flash Point (ºC): Undetermined
9. Specific optical rotation (º): Undetermined
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (kPa,25ºC): Undetermined3/mol):168.0
3. isotonic specific volume (90.2K):462.7
4. Surface Tension (dyne/cm):57.5
5. Polarizability(10-24cm3):24.79
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 2
3. Number of hydrogen bond acceptors: 3
4. Number of rotatable chemical bonds: 3
5. Number of tautomers: 4
6. Topological molecule polar surface area 49.3
7. Number of heavy atoms: 16
8. Surface charge: 0
9. Complexity: 236
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
None
Storage method
Should be sealed and stored in a cool, dry place. SPAN>
Synthesis method
will2gAnthranilic acid,3.2gbromobenzene,2gPotassium carbonate,0.1gLead powder and12mgNitrobenzene Mix , heating and reflux3h. Steam off the nitro group and unreacted bromobenzene, cool and filter, and use hydrochloric acid to make the filtrate acidic to obtain FONT-SIZE: 9pt; FONT-FAMILY: Arial “>N-Phenyl anthranilic acid crude product. The crude product was recrystallized from benzene and was 3gFinished product. SPAN>
Purpose
Reagent used to test vanadium in steel and determine potassium dichromate and vanadate. SPAN>
SPAN>2gPotassium carbonate,0.1gLead powder and12mgNitrobenzene Mix, Heating and reflow3h. Steam off the nitro group and unreacted bromobenzene, cool and filter, and use hydrochloric acid to make the filtrate acidic to obtain FONT-SIZE: 9pt; FONT-FAMILY: Arial “>N-Phenyl anthranilic acid crude product. The crude product was recrystallized from benzene and was 3gFinished product. SPAN>
Purpose
Reagent used to test vanadium in steel and determine potassium dichromate and vanadate. SPAN>
