2-Naphthylthiol
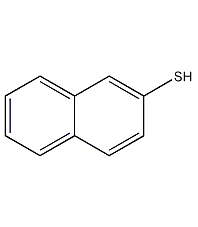

Structural formula
| Business number | 023R |
|---|---|
| Molecular formula | C10H18S |
| Molecular weight | 160.24 |
| label |
β-naphthalenethiophenol, 2-Naphthylthiol, 2-Naphthyl mercaptan, Thio-2-naphthol (β), C10H7SH |
Numbering system
CAS number:91-60-1
MDL number:MFCD00004086
EINECS number:202-082-5
RTECS number:QK3930000
BRN number:636389
PubChem number:24889151
Physical property data
1. Character:Light yellow crystalline powder.
2. Density (g/mL,25/4℃): Undetermined
3. Relative vapor density (g/mL,AIR=1): Undetermined
4. Melting point (ºC):81
5. Boiling point (ºC,Normal pressure):286
6. Boiling point (ºC, 1.33kpa):146.3
7. Refractive Index: Undetermined
8. Flash Point (ºC): Undetermined
9. Specific optical rotation (º): Undetermined
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (kPa,25ºC): Undetermined136.1
3. isotonic specific volume (90.2K):359.6
4. Surface Tension (dyne/cm):48.6
5. Polarizability(10-24cm3):20.72
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: 3
6. Topological molecule polar surface area 1
7. Number of heavy atoms: 11
8. Surface charge: 0
9. Complexity: 133
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
None
Storage method
Should be stored sealed in a cool and dark place.
Synthesis method
first2-Naphthol and dimethylaminothio Formyl chloride is prepared by reacting in the presence of a baseO-2 Naphthyl dimethylaminothiocarbamate, refined Heat to270-275℃, reaction45min. After cooling, add to the mixture of potassium hydroxide solution and ethylene glycol, and heat to reflux1h. After hydrochlorization, chloroform extraction, reduced imitation extraction, and vacuum distillation, you can obtain2-Naphthylthiol
Purpose
It is used as a regeneration activation agent in the rubber industry and is suitable for use at low temperatures. However, the activation effect is not as good as that of the mango disulfides, and the regeneration tape has an unpleasant smell. The general dosage is0.1-1.5% , this product can also be used as an antioxidant, plasticizer and intermediate for organic synthesis body. 2-Naphthothiol is toxic and can cause dermatitis and ulcers.
n'”>℃, reaction45min. After cooling, add the mixture of potassium hydroxide solution and ethylene glycol, Heated reflow1h. After hydrochlorization, chloroform extraction, reduced imitation extraction, and vacuum distillation, you can obtain 2-
Purpose
It is used as a regeneration activation agent in the rubber industry and is suitable for use at low temperatures. However, the activation effect is not as good as that of the mango disulfides, and the regeneration tape has an unpleasant smell. The general dosage is0.1-1.5% , this product can also be used as an antioxidant, plasticizer and intermediate for organic synthesis body. 2-Naphthothiol is toxic and can cause dermatitis and ulcers.
