2,3–dimethyl-1-butene
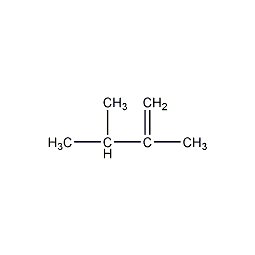

Structural formula
| Business number | 05T0 |
|---|---|
| Molecular formula | C6H12 |
| Molecular weight | 84.16 |
| label |
1-Methyl-1-isopropylethylene, hexacarbonene, 1-isopropyl-1-methylethylene, Dimethylbutene, Linear compounds and flavors |
Numbering system
CAS number:563-78-0
MDL number:MFCD00008923
EINECS number:209-262-2
RTECS number:None
BRN number:505982
PubChem number:24864727
Physical property data
1. Physical property data
1. Refractive index (nD20): 1.3896
2. Flash point (℃): -18
3 . Melting point (℃): -157.2
4. Boiling point (ºC): 56
5. Autoignition point (ºC): 360
6. Solubility: Insoluble in water, miscible in carbon disulfide, ethanol, and ether.
7. Toxicity: No toxicity data available.
8. Density (g/mL, 25/4℃):
9. Relative density (water = 1) 0.680 (20℃),
10. (Air = 1) 2.91
11. Solubility parameter (J·cm-3)0.5: 14.670 p>
12.van der Waals area (cm2·mol-1): 9.400×109
13.van der Waals volume (cm3·mol-1): 64.740
14. Liquid phase standard heat of combustion (enthalpy) ( kJ·mol-1): -3980.45
15. Liquid phase standard claims heat (enthalpy) (kJ·mol-1): -95.60
16. Liquid phase standard hot melt (J·mol-1·K-1): 184
17. Gas phase standard combustion heat (enthalpy) (kJ·mol-1): -4009.69
18. Gas phase standard claims heat (enthalpy) (kJ·mol-1): -66.36
19. Gas phase standard entropy (J·mol-1·K-1): 365.64
20. Gas phase standard free energy of formation (kJ·mol-1): 68.48
21. Gas phase standard hot melt (J·mol-1 sup>·K-1): 143.47
22. Flammable liquid, irritating
Toxicological data
None yet
Ecological data
3. Ecological data:
1. Other harmful effects: This substance may be harmful to the environment, and special attention should be paid to water bodies.
Molecular structure data
5. Molecular property data:
1. Molar refractive index: 29.37
2. Molar volume (cm3/mol): 122.8
3. Isotonic specific volume (90.2K): 253.4
4. Surface tension (dyne/cm): 18.1
5. Polarizability (10-24cm3): 11.64
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 2.8
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 0
4. Number of rotatable chemical bonds: 1
5. Number of tautomers: none
6. Topological molecule polar surface area 0
7. Number of heavy atoms: 6
8. Surface charge: 0
9. Complexity: 51.1
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
Stability and reactivity:
Incompatible materials: strong oxidizing agents.
Storage method
1. Storage
Store in a cool, ventilated warehouse. Keep away from fire and heat sources. Protect from direct sunlight. The warehouse temperature should not exceed 25℃. The lighting, ventilation and other facilities in the storage room should be explosion-proof, and the switches should be located outside the warehouse. It is prohibited to use mechanical equipment and tools that are prone to sparks. Fire and explosion prevention technical measures must be taken during tank storage. Equipped with the appropriate variety and quantity of fire equipment. When filling, attention should be paid to the flow rate (not exceeding 3m/s), and a grounding device should be installed to prevent the accumulation of static electricity. It should be stored separately from nitric acid, oxidants, etc. It should not be stored for a long time to avoid deterioration. When handling, load and unload with care to prevent damage to packaging and containers.
Synthesis method
None yet
Purpose
Uses:
Used in organic synthesis and also used as a comparison sample for chromatographic analysis.
