N-methylmethylamine hydrochloride
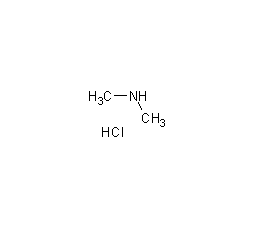
Structural formula
| Business number | 059J |
|---|---|
| Molecular formula | C2H8NCl |
| Molecular weight | 81.55 |
| label |
dimethylamine hydrochloride, Dimethylamine hydrochloride |
Numbering system
CAS number:506-59-2
MDL number:MFCD00012477
EINECS number:208-046-5
RTECS number:IQ0220000
BRN number:3589311
PubChem number:24847723
Physical property data
1. Character: white leaf-like crystal
2. Density (g/ cm3,25/4℃): Undetermined
3. Relative vapor density (g/cm3,AIR=1): Undetermined
4. Melting point (ºC):171
5. Boiling point (ºC,Normal pressure): Undetermined
6. Boiling point (ºC,8kPa): Undetermined
7. Refractive Index: Undetermined
8. Flash Point (ºF): Undetermined
9. Specific optical rotation (º): Undetermined
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (kPa,25ºC): Undetermined
Molecular structure data
1、 Molar refractive index:14.90
2、 Molar volume(m 3/mol):70.3
3, Isotonic specific volume ( 90.2K):139.6
4、 Surface tension(dyne /cm):15.5
5、 Polarizability(10 -24cm3):5.91
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 2
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: none
6. Topological molecule polar surface area 12
7. Number of heavy atoms: 4
8. Surface charge: 0
9. Complexity: 2.8
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 2
Properties and stability
Use and store according to specifications, no decomposition will occur, and avoid contact with oxides
Storage method
Save sealed and placed in a ventilated, dry environment
Synthesis method
It is obtained by forming a salt between dimethylamine and hydrochloric acid. Slowly drip hydrochloric acid into dimethylamine. The dosage of 30% hydrochloric acid is about 1.1 times that of 40% dimethylamine. The dripping speed is limited to a temperature of no more than 15°C and a pH of 7-8. After the reaction is completed, add activated carbon for decolorization and filter. The filtrate is adjusted to pH=3-4 with hydrochloric acid, and the water is evaporated under reduced pressure to obtain dimethylamine hydrochloride.
Purpose
1. Dimethylamine hydrochloride is a raw material for organic synthesis and is also used as a catalyst and magnesium reagent for acetylation analysis.
2. is used as a catalyst in acetylation analysis, and is also used in organic synthesis and the preparation of dimethylamine aqueous solution
3. Used as pharmaceutical raw materials
amily: Arial”>Surface tension(dyne/cm ):15.5
5、 Polarizability(10 -24cm3):5.91
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 2
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: none
6. Topological molecule polar surface area 12
7. Number of heavy atoms: 4
8. Surface charge: 0
9. Complexity: 2.8
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 2
Properties and stability
Use and store according to specifications, no decomposition will occur, and avoid contact with oxides
Storage method
Save sealed and placed in a ventilated, dry environment
Synthesis method
It is obtained by forming a salt between dimethylamine and hydrochloric acid. Slowly drip hydrochloric acid into dimethylamine. The dosage of 30% hydrochloric acid is about 1.1 times that of 40% dimethylamine. The dripping speed is limited to a temperature of no more than 15°C and a pH of 7-8. After the reaction is completed, add activated carbon for decolorization and filter. The filtrate is adjusted to pH=3-4 with hydrochloric acid, and the water is evaporated under reduced pressure to obtain dimethylamine hydrochloride.
Purpose
1. Dimethylamine hydrochloride is a raw material for organic synthesis and is also used as a catalyst and magnesium reagent for acetylation analysis.
2. is used as a catalyst in acetylation analysis, and is also used in organic synthesis and the preparation of dimethylamine aqueous solution
3. Used as pharmaceutical raw materials
Hydrochloride is a raw material for organic synthesis and is also used as a catalyst and magnesium reagent for acetylation analysis.
2. is used as a catalyst in acetylation analysis, and is also used in organic synthesis and the preparation of dimethylamine aqueous solution
3. Used as pharmaceutical raw materials
