thioacetic acid
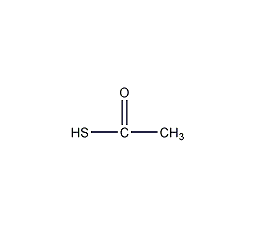

Structural formula
| Business number | 059T |
|---|---|
| Molecular formula | C2H4OS |
| Molecular weight | 76.12 |
| label |
Thiacetic acid, CH3COSH |
Numbering system
CAS number:507-09-5
MDL number:MFCD00004853
EINECS number:208-063-8
RTECS number:None
BRN number:1733298
PubChem number:24888974
Physical property data
1. Properties: Colorless and transparent liquid with pungent odor. [1]
2. Melting point (℃): -17[2]
3. Boiling point (℃): 93[3]
4. Relative density (water = 1): 1.07[4]
5. Relative vapor Density (air=1): 2.62[5]
6. Critical pressure (MPa): 6.92[6]
7. Octanol/water partition coefficient: 0.14[7]
8. Flash point (℃): 18~21[8]
9. Ignition temperature (℃): 427[9]
10. Explosion limit (%): 16[10] sup>
11. Lower explosion limit (%): 1.1[11]
12. Solubility: soluble in water, ethanol, ether, etc. [12]
Toxicological data
1. Acute toxicity[13] LD50: 200~400mg/kg (rat oral); 75mg/kg (small Mouse abdominal cavity)
2. Irritation No data available
Ecological data
This substance may be harmful to the environment, and special attention should be paid to water bodies.
Molecular structure data
1. Molar refractive index: 19.24
2. Molar volume (cm3/mol): 72.7
3. Isotonic specific volume (90.2K ): 168.6
4. Surface tension (dyne/cm): 28.9
5. Polarizability (10-24cm3): 7.62
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 0.3
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: 2
6. Topological molecule polar surface area 18.1
7. Number of heavy atoms: 4
8. Surface charge: 0
9. Complexity: 33
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Stability[14] Stable
2. Incompatible substances [15] Strong oxidants, strong bases
3. Conditions to avoid contact[16] Heating
4. Polymerization hazard[17] No polymerization p>
5. Decomposition products[18] Hydrogen sulfide
Storage method
Storage Precautions[19] Store in a cool, ventilated warehouse. Keep away from fire and heat sources. The storage temperature should not exceed 37°C. Keep container tightly sealed. They should be stored separately from oxidants, alkalis, and food chemicals, and avoid mixed storage. Use explosion-proof lighting and ventilation facilities. It is prohibited to use mechanical equipment and tools that are prone to sparks. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
Obtained from the co-thermal distillation of glacial acid and phosphorus pentathio. Add finely powdered phosphorus pentasulfide to glacial acetic acid. When heated to 91°C, thioacetic acid begins to evaporate. Bumping must be prevented during heating. The distillation temperature must not exceed 100°C. The distillate is re-distilled once to obtain the finished product.
Purpose
1. This product is an acetylthiolating agent and sulfhydrating agent in organic synthesis. It is mainly used in the synthesis of lipoic acid, cystine and mercaptocarboxylic acids. It is also used as a modifier and detoxifier of hormones. Synthesis of cephalosporin modifiers and bactericides, polymer modifiers and additives. Used in reagents, flavors and fragrances, cosmetics, synthetic captopril, etc.
2. Used as chemical reagents, organic synthesis reagents, tear gases, etc. [20]
