Thionyl bromide
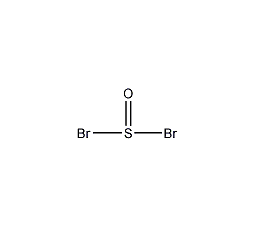
Structural formula
| Business number | 059U |
|---|---|
| Molecular formula | SOBr2 |
| Molecular weight | 207.87 |
| label |
Dibromide sulfoxide, thionyl bromide, brominating agent |
Numbering system
CAS number:507-16-4
MDL number:MFCD00011448
EINECS number:208-064-3
RTECS number:None
BRN number:None
PubChem number:24855121
Physical property data
1. Character: yellow liquid
2. Density (g/ cm3, 25/4℃): 2.672
3. Relative steam Density (g/cm3, air=1): Undetermined
4. Melting point (ºC): -49.5
5. Boiling point (ºC, Normal pressure): 47-49
6. Boiling point (ºC, 8kPa): Undetermined
7. Refractive index: Undetermined
8. Flash point (ºC): Undetermined
9. Specific optical rotation (º): Undetermined
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (kPa, 25ºC): 104
12. Saturated vapor pressure (kPa, 60ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Oil and water (octanol/ Log value of the distribution coefficient (water): Undetermined
17. Explosion upper limit (%, V/V): Undetermined
18. Explosion lower limit (%, V/V): Undetermined
19. Solubility: Reacts violently
Toxicological data
Acute toxicity:
Main irritant effects:
On skin: Causes corrosive effects on skin and mucous membranes.
On eyes: Strong corrosive effects
Tear-inducing effect.
Sensitization: No known sensitizing effects.
Ecological data
General remarks
Water hazard level 1 (German regulations) (self-assessment via list) This substance is slightly hazardous to water.
Do not allow undiluted or large amounts of product to come into contact with groundwater, waterways or sewage systems.
Do not discharge materials into the surrounding environment without government permission.
Molecular structure data
1. Molar refractive index: 26.37
2. Molar volume (cm3/mol): 64.4
3. Isotonic specific volume (90.2K ): 207.3
4. Surface tension (dyne/cm): 107.4
5. Polarizability (10-24cm3): 10.45
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 1.6
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: none
6. Topological molecule polar surface area 36.3
7. Number of heavy atoms: 4
8. Surface charge: 0
9. Complexity: 29
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
Use and store according to specifications, no decomposition will occur, and avoid contact with oxides
Storage method
Store in a cool, dry and well-ventilated warehouse at 2-8°C. Keep away from fire and heat sources. Protect from direct sunlight. The packaging is sealed. They should be stored separately from acids and food chemicals, and avoid mixed storage. Suitable materials should be available in the storage area to contain spills.
Synthesis method
1. Dissolve 50 grams (2.1 mol) of potassium austrine in 1500 ml of liquid sulfur dioxide in a bottle equipped with a dropping funnel and an air tube, and connect the air tube to In another bottle that is cooled to -80°C, the second bottle has an air guide tube leading to the exhaust port. 23.8 grams (1 mol) of thionyl chloride was added dropwise to the solution in the first bottle over 10 minutes to form a white precipitate. The sulfur dioxide was evaporated into a second bottle for another preparation. The reaction residue was kept at 20℃/10mm for 0.5 hours, and then thionyl bromide was pumped into a cold trap at 80℃ at 20℃/0.1mm, finally obtaining 21.7 grams of light yellow liquid (52%)
Thionyl bromide decomposes slowly and is best used immediately after preparation.
![]()
Purpose
Bromating agent. Preparation of bromide and acid bromide.
