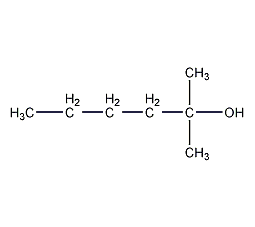
2-Methyl-2-hexanol
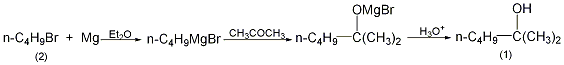
Structural formula
| Business number | 06RR |
|---|---|
| Molecular formula | C7H160 |
| Molecular weight | 116.20 |
| label |
1,1-dimethyl-1-pentanol, 1,1-Dimethyl-1-pentanol, Butydimethylcarbinol, 2-Methyl-2-hydroxyhexane, alcohol solvent |
Numbering system
CAS number:625-23-0
MDL number:MFCD00004486
EINECS number:210-881-5
RTECS number:None
BRN number:1697130
PubChem number:24847038
Physical property data
1. Properties: colorless, light yellow transparent liquid.
2. Density (g/mL, 25/4℃): 0.812
3. Relative density (20℃, 4℃): 0.8144
4 . Relative density (25℃, 4℃): 0.8103
5. Boiling point (ºC, normal pressure): 143
6. Refractive index at room temperature (n20
sup>): 1.4190
7. Refractive index (n20D): 1.4175
8. Flash point (ºC): 40
9. Specific rotation (º): Undetermined
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (kPa, 25ºC): Undetermined
12. Saturated vapor pressure (kPa, 60ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16 . The logarithmic value of the oil-water (octanol/water) partition coefficient: Undetermined
17. Explosion upper limit (%, V/V): Undetermined
18. Explosion lower limit (% ,V/V): Not determined
19. Solubility: Not determined.
Toxicological data
None
Ecological data
None
Molecular structure data
1. Molar refractive index: 35.97
2. Molar volume (cm3/mol): 141.6
3. Isotonic specific volume (90.2K ): 322.4
4. Surface tension (dyne/cm): 26.8
5. Polarizability (10-24cm3): 14.26
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 2
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 3
5. Number of tautomers: none
6. Topological molecule polar surface area 20.2
7. Number of heavy atoms: 8
8. Surface charge: 0
9. Complex…�:57.4
10. Number of isotope atoms: 0
11. Determined number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters :0
13. Determine the number of stereocenters of chemical bonds: 0
14. Uncertain number of stereocenters of chemical bonds: 0
15. Covalent bond units Quantity:1
Properties and stability
1. Keep away from fire sources.
2. Exist in mainstream smoke.
Storage method
Store in an airtight container in a cool, dry place. The storage place must be away from oxidants and sources of fire.
Synthesis method
Preparation method:
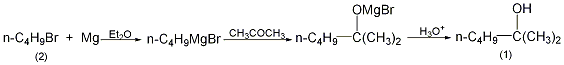
On In a dry reaction bottle equipped with a stirrer, thermometer, reflux condenser (installed with a calcium chloride drying tube) and dropping funnel, add 3.1g (0.13mol) magnesium strip, a small amount of crystallized iodine and 15mL anhydrous ether. Add dropwise a mixture of 17g (0.13mol) n-bromobutane (2) and 15mL anhydrous ether from the dropping funnel, adding about 5mL first. After a few minutes, it becomes slightly boiling. If there is no reaction, heat it slightly. After the reaction is stable, add 25 mL of anhydrous ether. The remaining mixture of butyl bromide and diethyl ether was added dropwise with stirring. Control the dropping speed to maintain a slight boil. After the addition is completed, the water bath is added to reflux for 15 minutes, and the reaction of metallic magnesium is basically complete. Under cooling in a water bath, slowly add 100 mL (10%) ethyl sulfuric acid dropwise (slowly in the initial stage, then gradually speed up) to decompose the product. After decomposition, separate the ether layer, extract the water layer with ether (25 mL × 2), combine the ether layers, wash with 30 mL (15%) carbon solution, and dry over anhydrous potassium carbonate. Evaporate the diethyl ether, then distill, and collect the fraction at 137-141°C to obtain 8g of 2-methyl-2-hexanol (1), with a yield of 53%. [1]
Purpose
None
