Diethyl bromomalonate
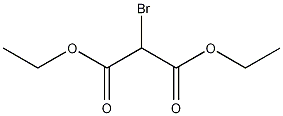

Structural formula
| Business number | 078Y |
|---|---|
| Molecular formula | C7H11BrO4 |
| Molecular weight | 239.06 |
| label |
Diethyl bromaleate, Diethyl bromomalonate, Bromomalonic Acid Diethyl Ester, BrCH(CO2CH2CH3)2, aliphatic compounds |
Numbering system
CAS number:685-87-0
MDL number:MFCD00009138
EINECS number:211-683-1
RTECS number:None
BRN number:509768
PubChem number:24894253
Physical property data
1. Properties: colorless liquid.
2. Density (g/mL, 25/4℃): 1.402
3. Relative vapor density (g/mL, air=1): Undetermined
4. Melting point (ºC): Undetermined
5. Boiling point (ºC, normal pressure): 233-235
6. Boiling point (ºC, 5.2kPa): Undetermined Determined
7. Refractive index: 1.451
8. Flash point (ºC): 111
9. Specific rotation (º): Undetermined
p>
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (kPa, 25ºC): Undetermined
12. Saturated vapor pressure (kPa, 60ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) partition coefficient: Undetermined
17. Explosion upper limit (%, V /V): Undetermined
18. Lower explosion limit (%, V/V): Undetermined
19. Solubility: Not soluble in water.
Toxicological data
None yet
Ecological data
Generally not hazardous to water, do not discharge material into the surrounding environment without government permission.
Molecular structure data
1. Molar refractive index: 45.71
2. Molar volume (cm3/mol): 164.6
3. Isotonic specific volume (90.2K ): 409.6
4. Surface tension (dyne/cm): 38.3
5. Dielectric constant:
6. Dipole moment (10-24cm3):
7. Polarizability: 18.12
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 1.8
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 4
4. Number of rotatable chemical bonds: 6
5. Number of tautomers:None
6. Topological molecule polar surface area 52.6
7. Number of heavy atoms: 12
8. Surface charge: 0
9. Complexity: 151
10. Number of isotope atoms: 0
11. Number of determined atomic stereocenters: 0
12. Uncertain atoms Number of stereocenters: 0
13. Determine the number of stereocenters of chemical bonds: 0
14. Uncertain number of stereocenters of chemical bonds: 0
15. Number of covalent bond units: 1
Properties and stability
Keep away from oxides and alkalis.
Storage method
Store in an airtight container in a cool, dry place. Store away from oxidizing agents.
Synthesis method
1. On the 1L three-necked flask, assemble a stirrer, a reflux condenser (with a conduit connected to the water bottle to absorb hydrogen bromide) and a separating funnel. Squeeze the end tube of the funnel so that it extends into the liquid and almost touches the blades of the stirrer. Place 160g (1mol) diethyl malonate and 150mL carbon tetrachloride in the bottle, and place 165g (53mL, 1.03mol) dry bromine in the separatory funnel. Add a few milliliters of bromine while stirring, place a large light bulb under the flask, and turn on the light bulb until the reaction begins. The remaining bromine is gradually added at a speed that makes the liquid boil slightly, and then refluxed until no more hydrogen bromide escapes (about
1h). Cool the mixture and wash 5 times with 5% sodium carbonate solution, 50 mL each time. Then distill under reduced pressure. Collect the distillates at 130℃/5.3kPa and 130~150℃/5.3kPa. The residue is about 20g. Redistill the low boiling point fractions and boil them together at 130~150℃/5.3kPa. The distillate is then distilled under reduced pressure, and the fraction at 132~136℃/4.4kPa is collected to obtain a product of 175~180g, with a yield of 73%~75%. Redistilling the low boiling point fraction can also add 15g of product.
2. Preparation method:
org/internal/day_121214/201212141106186886.gif” alt=”” />
It is equipped with a stirrer, a reflux condenser (connected to the hydrogen bromide absorption device), and a dropping funnel (the bottom extends into the liquid surface In the reaction flask below), add 160g (1.0mol) of diethyl malonate (2) and 150mL of carbon tetrachloride. Add 165g (1.03mol) of bromine to the dropping funnel. Add a few milliliters of bromine while stirring. A large light bulb shines until the reaction begins. Slowly add the remaining bromine dropwise at a rate that keeps the reaction solution slightly boiling. After the addition, continue to reflux for 1 hour until no more hydrogen bromide gas escapes. Cool and wash 5 times with 5% sodium carbonate solution, using approximately 50 mL each time. Distill under reduced pressure and collect the fractions at 130℃/5.3KPa and 130~150℃/5.3kPa respectively. Distill the 130℃/5.3KPa fraction again, combine the 130~150℃/5.3kPa fraction, re-distill, collect the 132~136℃/4.39kPa fraction, and obtain diethyl bromomalonate (1) 175 ~180g, yield 73% ~ 75%. [1]
Purpose
Organic reagents, pharmaceutical intermediates.
