Ethyl chloride
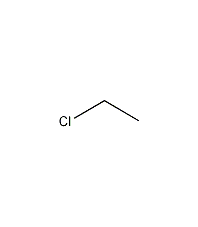
Structural formula
| Business number | 01HT |
|---|---|
| Molecular formula | C2H5Cl |
| Molecular weight | 64.51 |
| label |
Ethyl chloride, Ethyl chloride, Ethyl chloride, Aliphatic halogenated derivatives |
Numbering system
CAS number:75-00-3
MDL number:MFCD00000961
EINECS number:200-830-5
RTECS number:KH7525000
BRN number:1730751
PubChem number:24845449
Physical property data
1. Characteristics: colorless gas with an ether-like odor. [1]
2. Melting point (℃): -138.7[2]
3. Boiling point (℃): 12.5[3]
4. Relative density (water = 1): 0.92[4]
5. Relative vapor Density (air=1): 2.22[5]
6. Saturated vapor pressure (kPa): 133.3 (20℃)[6]
7. Heat of combustion (kJ/mol): -1323.8[7]
8. Critical temperature (℃): 187.2[8]
9. Critical pressure (MPa): 5.23[9]
10. Octanol/water partition coefficient: 1.43 [10]
11. Flash point (℃): -50 (CC) [11]
12. Ignition temperature ( ℃): 519[12]
13. Explosion upper limit (%): 14.8[13]
14. Explosion Lower limit (%): 3.6[14]
15. Solubility: Slightly soluble in water, miscible in most organic solvents. [15]
16. Viscosity (mPa·s, 5ºC, liquid): 0.292
17. Flash point (ºC, closed): -50
18. Flash point (ºC, opening): -43
19. Heat of evaporation (KJ/kg, b.p.): 383.0
20. Heat of fusion (KJ/kg): 69.04
21. Heat of formation (KJ/mol, liquid): 132.3
22. Heat of formation (KJ/mol, gas): 107.6
23. Specific heat capacity (KJ/(kg·K), 0ºC): 1.55
24. Electrical conductivity (S/m, 0ºC): <3×10-9
25. Thermal conductivity (W/(m·K), liquid): 0.14676
26. Volume expansion coefficient (K-1 , 0~15ºC, average): 0.00156
27. Refractive index at room temperature (n20): 1.36805
28 .Refractive index at room temperature (n25): 1.36625
29. Relative density (25℃, 4℃): 0.88895
30. Eccentricity factor: 0.204
31. Lennard-Jones parameter (A): 7.198
32. Lennard-Jones parameter (K) :206.3
33. Solubility parameter (J·cm-3)0.5:17.731
34.van der Waals Area (cm2·mol-1): 5.270×109
35. van der Waals volume (cm 3·mol-1): 35.520
36. Gas phase standard claims heat (enthalpy) (kJ·mol-1): -112.3
37. Gas phase standard entropy (J·mol-1·K-1): 275.89
38. Gas phase standard formation free energy (kJ·mol-1): -60.4
39. Gas phase standard hot melt (J·mol-1·K-1): 62.64
40. Liquid phase standard claims heat (enthalpy) (kJ·mol-1): -132.80
41. Liquid phase standard entropy (J·mol-1·K-1): 190.79
42. Liquid phase standard free energy of formation (kJ·mol-1): -55.73
43. Liquid phase standard hot melt (J·mol-1 sup>·K-1): 108.8
Toxicological data
1. Acute toxicity[16]
LC50: 160000mg/m3 (rat inhalation, 2h); 146000mg/kg (mouse inhalation)
2. Irritation No data available
3. Mutagenicity[17] Microbial mutagenicity: Salmonella typhimurium 10μg/dish. Mammalian somatic mutations: Hamster ovary 2340mg/L.
4. Carcinogenicity[18] IARC Carcinogenicity Comment: G3, insufficient evidence of carcinogenicity to humans and animals .
Ecological data
1. Ecotoxicity No data yet
2. Biodegradability[19]
Aerobic biodegradation (h): 168~672
Anaerobic biodegradation (h): 672~2688
3 .Non-biodegradability[20]
Photooxidation half-life in air (h): 160~1604
First-level hydrolysis half-life (h): 912
4. Other harmful effects[21] This substance has The environment may be hazardous and special attention should be paid to pollution of surface water, soil, atmosphere and drinking water, and special attention should be paid to aquatic life.
Molecular structure data
1. Molar refractive index: 16.16
2. Molar volume (cm3/mol): 72.9
3. Isotonic specific volume (90.2K ): 150.1
4. Surface tension (dyne/cm): 17.9
5. Polarizability (10-24cm3): 6.40
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 1.2
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 0
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: none
6. Topological molecule polar surface area 0
7. Number of heavy atoms: 3
8. Surface charge: 0
9. Complexity: 2.8
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. When there is no moisture, ethyl chloride will hardly change when heated to 400°C. It partially decomposes into ethylene and hydrogen chloride at 400~500℃. When heated to 500~600°C in the presence of pumice, most of it decomposes into ethylene and hydrogen chloride. Metals, metal chlorides and metal oxides can accelerate their decomposition. In alcohol-alkali solution, ethyl chloride easily removes hydrogen chloride to generate ethylene. Together with water, it is heated to 100°C in a sealed tube and hydrolyzed into ethanol. In the presence of catalysts such as titanium dioxide and barium chloride, it reacts with water vapor at 300~425°C to produce ethanol, acetaldehyde, and ethylene. It reacts with chlorine under light to produce 1,1-dichloroethane. In the presence of antimony pentoxide, 1,2-dichloroethane is generated. It reacts with benzene in the presence of Fridel-Crafts type catalyst to produce ethylbenzene. React with lead-sodium alloy to obtain tetraethyl lead.
2. Stability[22] Stable
3. Incompatible substances[23] Strong oxidants, potassium, sodium and their alloys
4. Polymerization hazards[24] No polymerization
5. Decomposition products[25] Hydrogen chloride
Storage method
Storage Precautions[26] Store in a cool, ventilated warehouse dedicated to flammable gases. Keep away from fire and heat sources. The storage temperature should not exceed 30℃. It should be stored separately from oxidants, active metal powders, etc., and avoid mixed storage. Use explosion-proof lighting and ventilation facilities. It is prohibited to use mechanical equipment and tools that are prone to sparks. The storage area should be equipped with leakage emergency response equipment.
Synthesis method
In the industrial production of ethyl chloride, the ethylene hydrochlorination method is generally used, a few use the ethane chlorination method, and a very few use the ethanol method.
1. Ethylene hydrochlorination method: produced by addition reaction of ethylene and hydrogen chloride as raw materials. The technology and economy of this method are relatively reasonable, and there are two types: gas phase method and liquid phase method. The liquid phase method is to react high-concentration ethylene with hydrogen chloride in a solvent such as ethyl chloride (30-40°C, 253-303kPa) in the presence of a catalyst such as AlCl3, and then perform gas-liquid separation after alkali washing, and distillation to obtain pure product. The gas phase method uses AlCl3, NH4Cl, silica gel, etc. as catalysts, uses a lower concentration of ethylene and ethane mixed gas as raw materials, and reacts at 130-250°C.
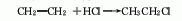
2. Ethane chlorination method : In industry, thermal chlorination is the main method, that is, ethane is chlorinated at 250-500℃ and pressure 202-304kPa (the by-product hydrogen chloride reacts with ethylene to produce ethyl chloride). 3. Ethanol method: react with ethanol and hydrochloric acid in the presence of zinc chloride. Raw material consumption quota: ethanol (95%) 945kg/t, hydrochloric acid (30%) 2830kg/t, zinc chloride 90kg/t. In addition, the tail gas after absorbing hydrochloric acid, a by-product of the production of trichloroacetaldehyde, is used as a 98%Ethyl chloride can also be obtained by drying with sulfuric acid, followed by pressurization, freezing, liquefaction, and distillation purification.
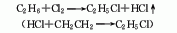
Purpose
1. Mainly used as raw material for tetraethyl lead, ethyl cellulose and ethyl carbazole dyes. It can also be used as aerosol, refrigerant, local anesthetic, ethylating agent, olefin polymerization solvent, gasoline anti-seismic agent, etc. It is also used as a catalyst for polypropylene and a solvent for phosphorus, sulfur, grease, resin, wax, etc.; in the synthesis of pesticides, dyes, medicines and their intermediates.
2. Used as a catalyst for polypropylene, and also used as a refrigerant, anesthetic, pesticide, etc. [27]
extended-reading:https://www.newtopchem.com/archives/40454extended-reading:https://www.newtopchem.com/archives/1041extended-reading:https://www.bdmaee.net/wp-content/uploads/2021/05/138-3.jpgextended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/Zinc-isooctanoate-CAS-136-53-8-Zinc-2-ethyloctanoate.pdfextended-reading:https://www.bdmaee.net/soft-foam-pipeline-composite-amine-catalyst/extended-reading:https://www.newtopchem.com/archives/39760extended-reading:https://www.cyclohexylamine.net/bismuth-neodecanoate-cas-251-964-6/extended-reading:https://www.bdmaee.net/monobutyl-tin-oxide/extended-reading:https://www.newtopchem.com/archives/45184extended-reading:https://www.cyclohexylamine.net/dabco-delay-type-catalyst-delay-type-strong-gel-catalyst/
