D-Mannitol D-Mannitol
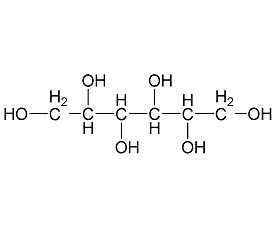
Structural formula
| Business number | 01FR |
|---|---|
| Molecular formula | C6H14O6 |
| Molecular weight | 182.17 |
| label |
Hexagonal alcohol, D-mannitol, D-xymelol, Mannitol, D-mannitol, cordycepic acid, Mannose mannose, mannitol, D-Mannite, Diosmol, Cordycepic acid, Manna suger, Mannite, sweetener, Aliphatic alcohols, ethers and their derivatives |
Numbering system
CAS number:69-65-8
MDL number:MFCD00064287
EINECS number:200-711-8
RTECS number:OP2060000
BRN number:1721898
PubChem number:24897341
Physical property data
1. Properties: colorless or white crystalline powder 2. Density (g/mL, 20/4℃): 1.52 3. Relative vapor density (g/mL, air=1): 1.489 4. Melting point (ºC): 132d 5. Boiling point (ºC, normal pressure): 292.53.5 6. Relative density (25℃, 4℃): 1.3433180.2 7. Relative Density (25℃, 4℃): 1.5398. Flash point (ºC, 3.5mmHg): 290-295
9. Specific rotation (º): 141º (c= USP-directives)
10. The standard heat of combustion (enthalpy) of the crystal phase (kJ·mol-1): -2813.0
11. The standard claim heat (enthalpy) of the crystal phase (kJ·mol -1): -1263.0
12. Saturated vapor pressure (kPa, 60ºC): Uncertain
13. Heat of combustion (KJ/mol): Uncertain
14. Critical temperature (ºC): Uncertain
15. Critical pressure (KPa): Uncertain
16. Oil and water (octanol/ Log value of the partition coefficient (water): Uncertain
17. Explosion upper limit (%, V/V): Uncertain
18. Explosion lower limit (%, V/V): Uncertain
19. Solubility: Easily soluble in hot water and glycerin, soluble in ethanol, pyridine and aniline.
Toxicological data
Acute toxicity: male intravenous LD50: 17143 mg/kg/2D-C; rat oral LD50: 13500 mg/kg; rat intravenous LD50: 9690 mg/kg; smallRat oral LC50: 22 mg/kg; mouse abdominal LC50: 14 mg/kg; mouse intravenous LC50: 7470 mg/kg.
Mutagenicity: Human Lymphocyte DNA Inhibition Test System: 50 mmol/L.
Ecological data
None
Molecular structure data
1. Molar refractive index: 38.89
2. Molar volume (cm3/mol): 114.1
3. Isotonic specific volume (90.2K ): 360.7
4. Surface tension (dyne/cm): 99.8
5. Polarizability (10-24cm3): 15.41
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 6
3. Number of hydrogen bond acceptors: 6
4. Number of rotatable chemical bonds: 5
5. Number of tautomers: none
6. Topological molecule polar surface area 121
7. Number of heavy atoms: 12
8. Surface charge: 0
9. Complexity: 105
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 4
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
The oral LC50 is 22000mg/kg for mice and 17300mg/kg for rats.
Storage method
Food grade plastic bag coated with kraft paper bag packaging. Store in a cool place
Synthesis method
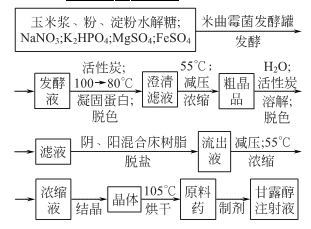
1. Mannitol can be extracted from kelp or seaweed, but electrolytic reduction or catalytic reduction with glucose or sucrose solution is more commonly used. Each is briefly introduced below.
(1) Extraction method from kelp: Add an appropriate amount of water to dried kelp and soak it at room temperature to swell, then stir continuously to dissolve the mannitol into the water. Use caustic soda to adjust the pH value of the washing water to above 12 and allow it to precipitate for more than 16H. Then use 1:1 sulfuric acid to adjust the pH value to neutral, then evaporate and concentrate until the relative density is 1.30~1.32 and then cool it. The supernatant liquid is then evaporated and concentrated until the relative density is 1.42~1.45. Thermal centrifugation removes salt and the concentrated solution is cooled. Crystallize and centrifuge to obtain crude mannitol. Recrystallize the crude product with boiling water, decolorize with activated carbon, elute chloride ions with alcohol, and dry to obtain pure mannitol.
(2) Electrolytic reduction method: Use glucose or sucrose as raw material to prepare a solution of appropriate concentration, adjust the pH value to 7.0 with sodium sulfate, and then add it to an electrolytic cell for electrolysis. The electrolyte is decolorized by activated carbon, vacuum concentrated, ethanol desalted, cooled and crystallized, and centrifuged to obtain crude mannitol. The crude mannitol is then recrystallized to obtain the fine product.
(3) Catalytic reduction method The catalytic reduction method is to process the sucrose solution through hydrolysis, neutralization, catalytic hydrogenation and other operations to obtain crude sorbitol and mannitol solutions, which are then filtered, activated carbon decolorized, ion exchanged and concentrated. , crystallization, separation and other operations to obtain high-quality mannitol.
2.Aspergillus oryzae fermentation method

3.Glucose produced by reducing sucrose hydrolysis It is then reduced with fructose to form a mixture of sorbitol and mannitol, which is then decolorized, separated and purified to obtain mannitol.
Purpose
1. Can be used as sweetener, anti-sticking agent, nutritional supplement, quality improver, and moisturizer.
2.Can be used in the plastics industry to produce rosin acid esters and artificial glycerin resins, explosives, detonators (nitrated mannitol), etc.; by hydrobromic acid The reaction can produce dibromomannitol.
3.Sweeteners, nutritional supplements, quality improvers, anti-sticking agents, etc. It has low calorie and low sweetness, and can be used as a special food for patients with diabetes and obesity instead of sugar. It is also an additive for chewing gum and hangover medicine.
4.Mainly used as a raw material in the pharmaceutical industry for the preparation of diuretics, dehydrating agents, and disaccharide substitutes , pharmaceutical excipients, etc. In terms of food, it is used as food for diabetics, bodybuilding food and low-calorie, low-sugar sweeteners. Industrially used in the plastics industry to make rosin acid esters and artificial glycerin resins, polyvinyl chloride plasticizers, explosives, etc. Also used in the cosmetics industry and toothpaste, etc.
