Benzothiophene 1-Benzothiophene
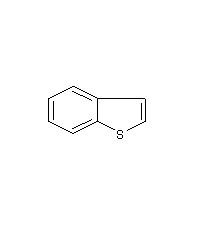

Structural formula
| Business number | 028P |
|---|---|
| Molecular formula | C8H6S |
| Molecular weight | 134.20 |
| label |
benzothiophene, thioindene, benzo[b]thiophene, thianaphthene, Thianaphthene, Thionaphthene |
Numbering system
CAS number:95-15-8
MDL number:MFCD00005864
EINECS number:202-395-7
RTECS number:None
BRN number:80580
PubChem number:24847823
Physical property data
1. Properties: White leaf-shaped crystals. Has a naphthalene-like odor. Can evaporate with water vapor.
2. Density (g/mL, 25/4℃): 1.1484
3. Relative vapor density (g/mL, air=1): Undetermined
4. Melting point (ºC): 32
5. Boiling point (ºC, normal pressure): 221
6. Boiling point (ºC, 5.2kPa): Undetermined
p>
7. Refractive index: 1.6374
8. Flash point (ºC): Undetermined
9. Specific rotation (º): Undetermined
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (kPa, 25ºC): Undetermined
12. Saturated vapor pressure (kPa , 60ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15 . . ): Undetermined
18. Lower explosion limit (%, V/V): Undetermined
p>
19. Solubility: Easily soluble in ethanol, soluble in ether, acetone and general organic solvents, insoluble in water. It turns cherry red when dissolved in concentrated sulfuric acid and disappears after heating.
Toxicological data
1. Acute toxicity: Rat oral LD50: 1700mg/kg
Rabbit transdermal LD5O: >2000mg/kg
Ecological data
This substance is harmful to the environment, and special attention should be paid to the pollution of water bodies.
Molecular structure data
1. Molar refractive index: 42.47
2. Molar volume (cm3/mol): 113.0
3. Isotonic specific volume (90.2K ): 294.2
4. Surface tension (dyne/cm): 45.9
5. Polarizability (10-24cm3): 16.84
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: none
6. Topological molecule polar surface area 28.2
7. Number of heavy atoms: 9
8. Surface charge: 0
9. Complexity: 101
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
It turns cherry red when dissolved in concentrated sulfuric acid and disappears after heating. Sulfonation reaction easily occurs. It turns light brown when exposed to light and air. Avoid contact with oxides.
Storage method
Stored sealed in a cool, dry place. Make sure the workspace has good ventilation. Keep sealed. Keep away from sources of fire and store away from oxidizing agents.
Synthesis method
In industry, it is mainly extracted from crude naphthalene. It can also be synthesized from styrene or ethylbenzene and hydrogen sulfide or produced by the condensation of thiophene and benzene rings.
Purpose
Manufacture of medicines and sulfur indigo.
