3,4-Dichlorotoluene 3,4-Dichlorotoluene
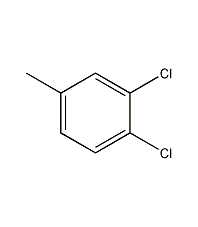

Structural formula
| Business number | 029R |
|---|---|
| Molecular formula | C7H6Cl2 |
| Molecular weight | 161.03 |
| label |
1,2-Dichloro-4-toluene, 3,4-Dichlorotoluene (dichlorotoluene), 1,2-Dichloro-4-methyl-benzene, CH3C6H3Cl2, Halogenated hydrocarbon solvents, aromatic compounds |
Numbering system
CAS number:95-75-0
MDL number:MFCD00000556
EINECS number:202-447-9
RTECS number:None
BRN number:1931687
PubChem number:24849924
Physical property data
1. Properties: colorless liquid with pungent odor. [1]
2. Melting point (℃): -15.3[2]
3. Boiling point (℃): 208.9[3]
4. Relative density (water = 1): 1.26[4]
5. Octanol /Water partition coefficient: 3.95[5]
6. Flash point (℃): 85.56[6]
7 .Ignition temperature (℃): 450[7]
8. Solubility: insoluble in water, miscible in ethanol, ether, acetone, benzene, tetrachloride carbon. [8]
Toxicological data
1. Acute toxicity No data available
2. Irritation No data available
Ecological data
1. Ecotoxicity[9] LC50: 5mg/L (7d) (fish)
2. Biodegradability No information available
3. Non-biodegradability No information available
Molecular structure data
1. Molar refractive index: 40.86
2. Molar volume (cm3/mol): 129.6
3. Isotonic specific volume (90.2K ): 316.6
4. Surface tension (dyne/cm): 35.6
5. Polarizability: 16.20
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 0
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: none
6. Topological molecule polar surface area 0
7. Number of heavy atoms: 9
8. Surface charge: 0
9. Complexity: 92.9
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. It can burn when exposed to open fire and decompose under high heat to produce toxic and corrosive smoke.
2. Stability[10] Stable
3. Incompatible substances[11] Strong oxidizing agent, strong alkali
4. Conditions to avoid contact[12] Heating
5. Aggregation hazard[13] No polymerization
6. Decomposition products[14] Hydrogen chloride
Storage method
Storage Precautions[15] Stored in a cool, ventilated warehouse. Keep away from fire and heat sources. Keep container tightly sealed. They should be stored separately from oxidants, alkalis, and food chemicals, and avoid mixed storage. Equipped with the appropriate variety and quantity of fire equipment. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
None
Purpose
1. Used as solvent and in organic synthesis. [16]
