Sodium salicylate Sodium salicylate
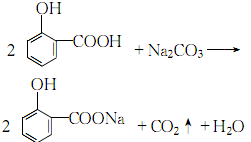
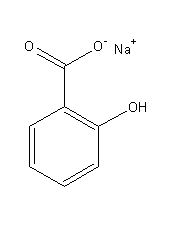
Structural formula
| Business number | 016A |
|---|---|
| Molecular formula | C7H5NaO3 |
| Molecular weight | 160.1 |
| label |
Sodium o-hydroxybenzoate, 2-Hydroxybenzoic acid monosodium salt, sodium salicylate, Sodium Cylate, Sodium 2-Hydroxybenzoate, 2-Hydroxybenzoic acid sodium salt, Salicylic acid sodium salt, preservative |
Numbering system
CAS number:54-21-7
MDL number:MFCD00002440
EINECS number:200-198-0
RTECS number:VO5075000
BRN number:3732792
PubChem number:24899540
Physical property data
1. Properties: White scaly crystals or powder. Odorless. It turns pink when exposed to light.
2. Density (g/mL, 25/4℃): Undetermined
3. Relative vapor density (g/mL, air=1): Undetermined
4. Melting point (ºC): 160~166
5. Boiling point (ºC, normal pressure): Undetermined
6. Boiling point (ºC, 5.2kPa): Undetermined
7. Refractive index: Undetermined
8. Flash point (ºC): Undetermined
9. Specific rotation (º): Undetermined Determined
10. Autoignition point or ignition temperature (ºC): Not determined
11. Vapor pressure (kPa, 25ºC): Not determined
12. Saturated vapor pressure (kPa, 60ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) partition coefficient: Undetermined
17. Explosion upper limit ( %, V/V): Undetermined
18. Lower explosion limit (%, V/V): Undetermined
19. Solubility: Easily soluble in water, ethanol, and glycerin. Almost insoluble in ether, chloroform and benzene. 1g product is dissolved in 0.9ml water, 9.2ml ethanol and 4ml glycerol. The aqueous solution is slightly acidic, with a pH of 5 to 6.
Toxicological data
Ecological data
None
Molecular structure data
None
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 3
4. Number of rotatable chemical bonds: 1
5. Number of tautomers: 4
6. Topological molecule polar surface area 60.4
7. Number of heavy atoms: 11
8. Surface charge: 0
9. Complexity: 138
10. Number of isotope atoms: 0
11. Determine the atomic stereocenter Quantity: 0
12. Uncertain number of stereocenters of atoms: 0
13. Determined number of stereocenters of chemical bonds: 0
14. Uncertain chemical bonds Number of stereocenters: 0
15. Number of covalent bond units: 2
Properties and stability
1. Turns pink when exposed to light.
Storage method
Keep away from light.
Synthesis method
1. Add salicylic acid and sodium bicarbonate alternately into distilled water, keep the temperature at 60°C, and keep it in an acidic state. At the same time, add an appropriate amount of ethylenediaminetetraacetic acid (EDTA) and insurance powder. Raise the temperature to 85°C and react for half an hour. The qualified reaction liquid is filtered and sent to the ebullating bed, and dried at 85°C to obtain salicylic acid.
2.Dissolve salicylic acid in distilled water at 50°C, and slowly neutralize it with dilute sodium hydroxide to pH while stirring. Value 5.4~5.8:
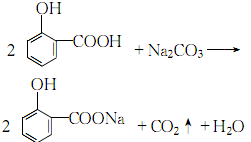
After the reaction, decolorize and filter. After the filtrate is clear, concentrate it under reduced pressure to a slurry, cool it, filter out the crystals, and dry it to get the finished product.
Purpose
1. Organic synthetic raw materials, preservatives, and reagents for measuring free acid in gastric juice. Antipyretics, analgesics and antirheumatic drugs. 2. Used as analytical reagent. Also used in organic synthesis, medicine, electronics, instrumentation, metallurgical industry, etc.
