Neopenty alcohol Neopenty alcohol
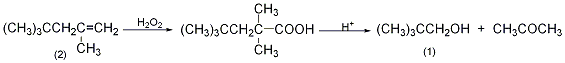
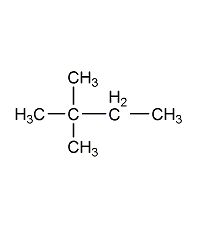
Structural formula
| Business number | 01K8 |
|---|---|
| Molecular formula | C5H12O |
| Molecular weight | 88.15 |
| label |
2,2-Methyl-1-propanol, tert-butylmethanol, tert-Butyl carbinol, 2,2-Dimethylpropanol, Neopentanol, Neopentyl alcohol, alcohol solvents, aliphatic compounds |
Numbering system
CAS number:75-84-3
MDL number:MFCD00004682
EINECS number:200-907-3
RTECS number:None
BRN number:1730984
PubChem number:24865983
Physical property data
1. Properties: colorless crystals with mint smell.
2. Density (g/mL, 20℃): 0.811
3. Solubility parameter (J·cm-3)0.5 : 19.265
4. Melting point (ºC): 52.5
5. Boiling point (ºC, normal pressure): 113~114
6. van der Waals area (cm2·mol-1): 9.170×109
7. Refractive index ( 50ºC): 1.3915
8. Flash point (ºC, closed): 36
9. van der Waals volume (cm3·mol -1): 62.610
10. Gas phase standard entropy (J·mol-1·K-1): 366.85 p>
11. Liquid phase standard combustion heat (enthalpy) (kJ·mol-1): -3283.2
12. Liquid phase standard claimed heat (enthalpy) ( kJ·mol-1): -399.4
13. Liquid phase standard entropy (J·mol-1·K-1): 229.3
14. Liquid phase standard free energy of formation (kJ·mol-1): -175.23
15. Critical pressure ( KPa): Undetermined
16. Log value of oil-water (octanol/water) partition coefficient: Undetermined
17. Explosion upper limit (%, V/V): Undetermined
18. Lower explosion limit (%, V/V): Undetermined
19. Solubility (%, water, 20ºC): 0.039
20. Dissolution Properties: Slightly soluble in water, miscible with many organic solvents such as alcohols, ethers, ketones, esters and aromatic hydrocarbons, and also miscible with mineral oil and vegetable oil.
Toxicological data
None
Ecological data
None
Molecular structure data
1. Molar refractive index: 26.71
2. Molar volume (cm3/mol): 108.6
3. Isotonic specific volume (90.2K ):242.9
4. Surface tension (dyne/cm): 25.0
5. Polarizability (10-24cm3) :10.59
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 1
5. Number of tautomers: none
6. Topological molecule polar surface area 20.2
7. Number of heavy atoms: 6
8. Surface charge: 0
9. Complexity: 33.7
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. It has the chemical reactivity of primary alcohols. Highly flammable. When using, avoid inhaling the dust of this product and avoid contact with eyes and skin.
2. Exist in smoke.
Storage method
This product should be sealed and stored in a cool place.
Synthesis method
1. Preparation method:
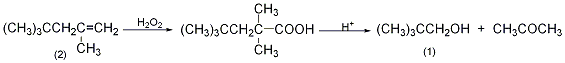
In a reaction bottle equipped with a stirrer, thermometer, and dropping funnel, add 800g of 30% hydrogen peroxide, cool it in an ice bath, and add dropwise a dilute solution composed of 800g of concentrated sulfuric acid and 310g of crushed ice while stirring and cool it to below 10°C. For sulfuric acid, control it at 5-10°C and finish adding it in about 20 minutes. Then, 224.4g (2.0mol) of 2,4,4-trimethyl-1-pentene (2) was added dropwise, and the addition was completed in 5 to 10 seconds. Remove the ice bath and stir the reaction at 25°C for 24 hours. Separate the organic layer and cool it in an ice bath, add 500g of 70% sulfuric acid dropwise with vigorous stirring, and keep the internal temperature at 15-25°C, which will take about 67-75 minutes. After the addition is completed, stir at 5 to 10°C for 30 minutes. Leave to stand for 1 to 3 hours, separate the organic layer, pour into 1000 mL of water, and distill under normal pressure (foam may appear, and distillation can be stopped at this time). After cooling the distilled liquid, separate the organic layer, dry it over anhydrous sodium sulfate①, fractionate, collect the fractions between 111 and 113°C to obtain 2,2-dimethyl-1-propanol ②(1) 60~70, yield 34%~40%. Note: ① Dry thoroughly before distillation, otherwise the product will form an azeotrope (80~85℃) with water, which will affect the yield. ② This reaction is similar to the hydrogen peroxide oxidation of ethyl-propyl benzene to produce phenol and acetone. Under acidic conditions, the peroxide is rearranged to produce alcohol and acetone. [1]
Purpose
Solvent, raw material for organic synthesis.
