p-Chlorotrifluorotoluene 1-Chloro-4-(trifluoromethyl)benzene
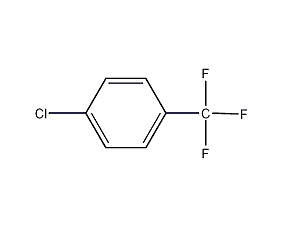
Structural formula
| Business number | 02E4 |
|---|---|
| Molecular formula | C7H4ClF3 |
| Molecular weight | 180 |
| label |
p-chlorotrifluorobenzyl, 4-Chlorotrifluorotoluene, 1-Chloro-4-(trifluoromethyl)benzene, p-Chlorotrifluoromethylbenzene, p-chlorobenzyltrifluoride, p-Trifluoromethyl chlorobenzene, p-Chlorotrifluoromethyl benzene, Pesticide intermediates; aromatic halogen derivatives |
Numbering system
CAS number:98-56-6
MDL number:MFCD00000627
EINECS number:202-681-1
RTECS number:XS9145000
BRN number:510203
PubChem number:24854225
Physical property data
1. Properties: Colorless and transparent liquid at room temperature.
2. Relative density (g/mL, 20/4℃): 1.342
3. Relative vapor density (g/mL, air=1): 6.24
4. Melting point (ºC): -36
5. Boiling point (ºC): 139
6. Refractive index (20ºC): 1.447
7. Flash point (ºC): 47
8. Solubility: Insoluble in water, soluble in alcohol, ether, benzene, toluene and other organic solvents.
9. Refractive index at room temperature (n25): 1.443030
10. Relative density (25℃, 4℃ ): 1.327630
Toxicological data
1. Acute toxicity: Rat oral LD50: 13mg/kg; Rat inhalation LC50: 22mg/m3; Mouse oral LD50: 11500mg/kg; Mouse inhalation LC50: 20mg/m 3; 2. Other multiple dose toxicity: Rat oral TDLo: 5600 mg/kg/14D-I; Rat oral TDLo: 28 mg/kg/28D-I; Rat inhaled TCLo : 440mg/m3/24H/17W-C; Rat inhalation TCLo: 500ppm/6H/4W-I; Mouse oral TDLo: 14mg/kg/14D-I; 3. Mutagenicity: Non-qualitative DNA comprehensive test: Human embryo, 1mg/L; 4. Irritating to eyes, respiratory system and skin.
Ecological data
This substance is harmful to the environment, and special attention should be paid to the pollution of water bodies.
Molecular structure data
1. Molar refractive index: 36.12
2. Molar volume (cm3/mol): 134.8
3. Isotonic specific volume (90.2K ): 300.3
4. Surface tension (dyne/cm): 24.5
5. Dielectric constant:
6. Dipole moment (10-24cm3):
7. Polarizability: 14.32
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 3
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: none
6. Topological molecule polar surface area 0
7. Number of heavy atoms: 11
8. Surface charge: 0
9. Complexity: 124
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Avoid contact with strong oxidants and strong alkali. Flammable.
2.Toxic. Toxic and irritating if inhaled. There is a danger of burning when exposed to high temperatures, open flames, or oxidants.
Storage method
1. Store in a cool, ventilated warehouse. Keep away from fire and heat sources. Protect from direct sunlight. The storage temperature should not exceed 30℃. Keep container tightly sealed. They should be stored separately from oxidants, alkalis, and food chemicals, and avoid mixed storage. Use explosion-proof lighting and ventilation facilities. It is prohibited to use mechanical equipment and tools that are prone to sparks. The storage area should be equipped with emergency release equipment and suitable containment materials.
2. This product is irritating. It is packed in galvanized iron barrels with a net weight of 200kg. It should be stored in a ventilated, sun-proof and moisture-proof place
Synthesis method
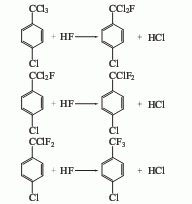
1. Obtained from p-chlorotoluene through side-chain photochlorination and liquid-phase fluorination: Chlorination: Put p-chlorotoluene into the photochlorination reactor, heat to 120-150°C, and pass under fluorescent lamp irradiation Chlorine reaction. Take a trace amount of the reaction solution every 10-15 minutes for gas chromatography analysis. Stop the reaction when the p-chlorotrichlorotoluene content is greater than 90%. The product is distilled under reduced pressure to obtain p-chlorotrichlorotoluene. The yield is 90-95%. Fluorination: Put p-chlorotrichlorotoluene into the fluorination reaction kettle, and add 4-5 times anhydrous hydrogen fluoride at a molar ratio. After raising the temperature, react at 1.9-2.4MPa until the reaction pressure no longer rises. The hydrogen chloride gas released during the reaction is absorbed by alkali, and the reactants are neutralized and steam distilled to obtain crude products. Then distill under normal pressure to obtain p-chlorotrifluorotoluene liquid with a boiling range of 138-140°C. The yield is 80-90%.
2. The preparation method is to add parachlorotrichlorotoluene into the fluorination reactor, cool the condenser with frozen brine, and press the measured liquid hydrogen fluoride from the cylinder to the fluorination reactor with dry air. , then add the catalyst, close the regulating valve, stir, and heat up the reaction. The hydrogen fluoride produced by the reaction condenses and refluxes along with the volatilized hydrogen fluoride and organic matter. The liquid hydrogen fluoride recovered by condensation is stored in the recovery tank. After the reaction, the fluorinated product p-chlorotrifluorotoluene enters The distillation kettle is heated and distilled under a vacuum system to obtain the product.

Purpose
Used in the manufacture of dyes, pigments, drugs, pesticides, etc. Solvents, dye intermediates.
It is the synthetic herbicide trifluralin, acifluorfen, oxyfluorfen (2-chloro-4-trifluoromethylphenyl-4′-nitro-3′-ethyl Oxyphenyl ether) and other intermediates.
