Phytic acid Phytic acid
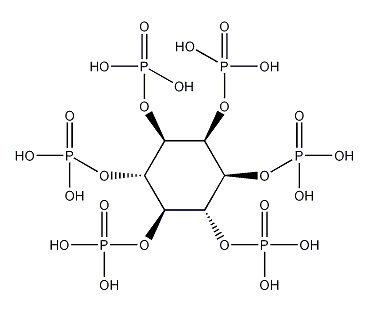
Structural formula
| Business number | 01UB |
|---|---|
| Molecular formula | C6H18O24P6 |
| Molecular weight | 660.04 |
| label |
phytic acid, Phytate, cyclohexanol phosphate, Myo-inositol hexakis(dihydrogen phosphate), 1,2,3,4,5,6-Cyclohexanehexol phosphoric aicd, Antioxidants, food additives, pH regulator, color protectant, anti-explosion agent, antistatic agent, Preservatives for pasta |
Numbering system
CAS number:83-86-3
MDL number:MFCD00082309
EINECS number:201-506-6
RTECS number:NM7525000
BRN number:2201952
PubChem number:24887537
Physical property data
1. Properties: It is a light yellow to tan syrupy liquid.
2. Density (g/mL, 25/4℃): 1.283
3. Relative vapor density (g/mL, air=1): Undetermined
4. Melting point (ºC): Undetermined
5. Boiling point (ºC, normal pressure): 105℃
6. Boiling point (ºC, 5.2kPa): Undetermined
7. Refractive index: 1.391
8. Flash point (ºC): Undetermined
9. Specific rotation (º): Undetermined
p>
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (kPa, 25ºC): Undetermined
12. Saturated vapor pressure (kPa, 60ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) partition coefficient: Undetermined
17. Explosion upper limit (%, V /V): Undetermined
18. Lower explosion limit (%, V/V): Undetermined
19. Solubility: Easily soluble in water, ethanol and acetone, difficult to dissolve In anhydrous ethanol, ether, benzene, hexane and chloroform.
Toxicological data
Acute toxicity:
Mouse caliber LC50: 500mg/kg
Rabbit caliber LDLO: 45mg/kg
Mouse oral LC50: 4192mg/ kg (50% phytic acid aqueous solution)
Ecological data
None yet
Molecular structure data
1.Molar refractive index: 96.96
2. Molar volume (cm3/mol): 272.7
3. Isotonic specific volume (90.2K): 982.7
4. Surface tension (dyne/cm): 168.6
5. Polarizability (10-24cm3): 38.43
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): -10.3
2. Number of hydrogen bond donors: 12
3. Number of hydrogen bond acceptors: 24
4. Number of rotatable chemical bonds: 12
5. Number of tautomers: none
6. Topological molecular polar surface area 401
7. Number of heavy atoms: 36
8. Surface charge: 0
9. Complexity: 818
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. It decomposes when exposed to high temperatures and has strong chelating ability.
2.Phytic acid obtained from rice bran has extremely low toxicity. The oral LC50 of mice is 4.9g/kg, which is lower than lactic acid.
Storage method
1. This product should be sealed and stored in a cool, dry place.
2. Generally packed in polyethylene plastic barrels, the weights are divided into 1kg, 10kg and 20kg. Store and transport according to general chemical regulations.
Synthesis method
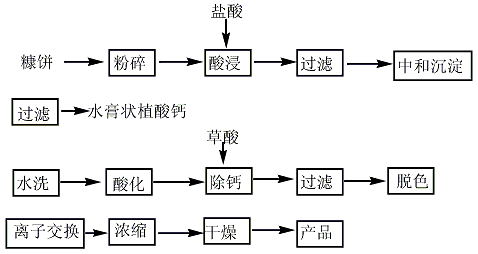
(1) It exists widely in nature, but almost never exists in a separate free state. Generally, they are widely present in plants in the form of complex salts of calcium, magnesium or potassium (calcium magnesium phosphate hexaphosphate) and proteins. They are often found in seeds, grains, germs and rice bran. There are many literature reports on the preparation method of this product. Rice bran or wheat bran is used as raw material, soaked in dilute acid, filtered, neutralized and precipitated with lime and sodium hydroxide, and then acidified and exchanged with ion exchange resin, concentrated under reduced pressure, decolorized and filtered to obtain the finished product. In addition, it can also be chemically synthesized through cyclohexanol and inorganic phosphoric acid, or by using calcium and magnesium phytate as raw materials to remove metal ions. It can also be extracted from corn activated sludge.
(2) Soak the broken rice bran in dilute hydrochloric acid with a Ph value of 2 at 50-60°C for 4-6 hours. After filtering, soak the filter residue for another 2 hours, and filter the discarded residue; combine the two filtrates. Let it stand for 10 hours, and absorb the supernatant; add an appropriate amount of Ca(OH)2 and Mg(OH)2 to the clear solution, and use NaOH solution to adjust the Ph value from 3.4 to 7.0. Stir for 15 minutes and let it stand for 2 to 3 hours; discard the supernatant. The clear liquid is filtered, and the filter residue is washed with an alkali aqueous solution with a Ph value of 7.5 and distilled water in sequence to obtain calcium and magnesium phytate salts with a yield of 95% to 98%.
Dissolve the calcium and magnesium phytate salts in a dilute hydrochloric acid solution with a Ph value of 3, soak and stir at 75°C for 1 hour, and maintain the Ph value at 3.5-4.5 to dissolve the calcium and magnesium phytate salts and precipitate protein. Add diatomaceous earth with 1% of the weight of the supernatant, stir and let it stand for suction filtration to remove protein and other precipitates; the filtrate passes through a strong acidic cation exchange resin and a strong basic anion exchange resin in sequence to obtain a dilute phytic acid solution; reduce the temperature at 75°C. Press and concentrate until the content is 55% to 65%.
Put the above phytic acid solution into a polyol solvent (ethylene glycol, propylene glycol or glycerin, etc.), heat and reflux for hydrolysis at 130-150°C for 3-4 hours, and adjust the Ph of the hydrolyzate at 100°C ± 10°C. The value is 8 to 9, then keep warm and stir for 1 hour, let it stand for filtration, heat the filtrate to 135°C to dehydrate, then add 3 times the amount of absolute ethanol, and let it stand to precipitate the crystallized product.

Purpose
1. It can be used as chelating agent, antioxidant, water softener, metal anti-corrosion and anti-rust agent, electroplating brightener and feed additive, etc. It is widely used in food, daily chemical, pharmaceutical, chemical, anti-corrosion and other industries. 2.Phytic acid is widely found in plant seeds and is a natural nutritional product. Its most notable feature is its extremely strong complexing effect with metal ions and its antioxidant properties. Can remove excessive metal ions harmful to the body. Used as antioxidant in fruit and vegetable products, fruit and vegetable juice drinks, edible oils and meat products. 3.In cosmetics, it can improve skin color (can inhibit tyrosinase) and promote skin blood circulation, and can be used to prepare moisturizers. It can also be used in anti-dandruff shampoos, hair dyes, etc. It can also be used in toothpaste, mouthwash, dental cement, and tooth filling cleaner. Add to toothpaste formula at 1% dosage to prevent bloating. It can also be used as a cleaning agent for chelated calcium salts.
4. In the food industry, it is used as an antioxidant for oils, a preservative for bean sprouts and other vegetables, a preservative for pasta, a flavor enhancer and a discoloration preventer for soy sauce and pickled products.
