Citrazinic acid Citrazinic acid
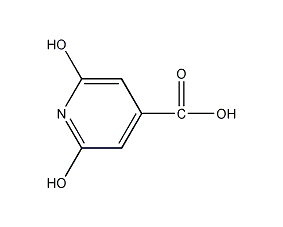

Structural formula
| Business number | 02F9 |
|---|---|
| Molecular formula | C6H5NO4 |
| Molecular weight | 155.11 |
| label |
2,6-Dihydroxyisonicotinic acid, dihydroxyazobenzoic acid, Bis-amide citric acid, 2,6-Dihydroxy-4-pyridinecarboxylic acid, 2,6-Dihydroxy-4-pyridinecarboxylic acid, 2,6-Dihydroxyisonicotinic acid |
Numbering system
CAS number:99-11-6
MDL number:MFCD00006274
EINECS number:202-731-2
RTECS number:NS1400000
BRN number:383736
PubChem number:24849298
Physical property data
1. Properties: light yellow needle-like crystals or powder
2. Density (g/mL, 25℃): Undetermined
3. Relative vapor density (g/ mL, air=1): Undetermined
4. Melting point (ºC): >300
5. Boiling point (ºC, normal pressure): Undetermined
6. Boiling point (ºC, 30mmHg): Not determined
7. Refractive index: Undetermined
8. Flash point (ºC):
9. Specific rotation (º): Undetermined
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (mmHg, 119ºC): Undetermined
12. Saturated vapor pressure (kPa, 119.3ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) partition coefficient:
17. Explosion upper limit (%, V/V): Undetermined
18. Explosion lower limit (%, V/V): Undetermined
19. Solubility: Almost Insoluble in cold water.
Toxicological data
Acute toxicity: Rat oral LD50: >3200mg/kg; Rat peritoneal cavity LDL0: 800mg/kg; Guinea pig skin contact LD50: >20mL/kg;
Ecological data
This substance is slightly hazardous to water.
Molecular structure data
1. Molar refractive index: 35.03
2. Molar volume (cm3/mol): 92.0
3. Isotonic specific volume (90.2K ): 293.5
4. Surface tension (dyne/cm): 103.4
5. Dielectric constant:
6. Dipole moment (10-24cm3):
7. Polarizability: 13.88
Compute chemical data
1��� Reference value for hydrophobic parameter calculation (XlogP): -0.7
2. Number of hydrogen bond donors: 3
3. Number of hydrogen bond acceptors: 4
4. Number of rotatable chemical bonds: 1
5. Number of tautomers: 2
6. Topological molecular polar surface area (TPSA): 86.6
7. Number of heavy atoms: 11
8. Surface charge: 0
9. Complexity: 276
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters : 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
Avoid contact with strong oxidizing agents.
Storage method
Store in a cool, ventilated warehouse. Keep away from fire and heat sources. The packaging is sealed. should be kept away from oxidizer, do not store together. Equipped with the appropriate variety and quantity of fire equipment. Suitable materials should be available in the storage area to contain spills.
Synthesis method
It is obtained from citric acid through esterification, aminolysis and ring closure. The esterification reaction is carried out in the presence of sulfuric acid. After 48 hours of dehydration, the solvent and ethanol are recovered. The residue is washed with water, alkali, and water until the pH is 6 to obtain crude triethyl citrate. Add ammonia water and pass ammonia until saturated. After standing, saturate with ammonia again, saturate once a day for three times in total, filter out the crystals, incubate the obtained triformamide citrate at about 120°C for 3 hours in the presence of sulfuric acid, slowly pour it into distilled water, and obtain the crude product. Refined to obtain citracinic acid. The preparation method using trimethyl citrate is roughly the same as the above process. Add ammonia water to trimethyl citrate and leave it at room temperature for about 48 hours. After concentration, add 75% sulfuric acid and leave it for 12 hours. Heat at 130°C for 10 minutes. Dilute with water and filter the precipitate to obtain the crude product. Dissolve the crude product in ammonia water, add hydrochloric acid to obtain precipitation, filter, wash with water and dry. The yield is 69%.
Purpose
Used in the synthesis of drugs, dyes and color film couplers.
