Trehalose D-Trehalose
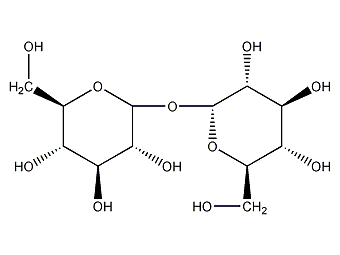

Structural formula
| Business number | 02FD |
|---|---|
| Molecular formula | C12H22O11 |
| Molecular weight | 342.3 |
| label |
mushroom sugar, Anhydrous trehalose, α-D-glucopyranosyl-α-D-glucopyranoside, D-trehalose anhydrous, Trehalose, Trehalose anhydrous, sweetener |
Numbering system
CAS number:99-20-7
MDL number:MFCD00006628
EINECS number:202-739-6
RTECS number:None
BRN number:None
PubChem ID:None
Physical property data
1. Properties: Colorless crystals, easily absorb moisture to form hydrates with two molecules of water, odorless, slightly sweet taste. 2. Density (g/mL, 25℃): Undetermined
3. Relative vapor density (g/mL, air=1): Undetermined
4. Melting point (ºC): 96.5~ 97.5℃ (dihydrate); 210.5℃ (anhydrous)
5. Boiling point (ºC, normal pressure): Undetermined
6. Boiling point (ºC, 98.5KPa) : Undetermined
7. Refractive index: Undetermined
8. Flash point (ºC): Undetermined
9. Specific rotation (º): +178
10. Autoignition point or ignition temperature (ºC): Not determined
11. Vapor pressure (mmHg, 119ºC): Not determined
12 . Saturated vapor pressure (kPa, 119.3ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) partition coefficient:
17. Explosion upper limit ( %, V/V): Undetermined
18. Lower explosion limit (%, V/V): Undetermined
19. Solubility: soluble in water, insoluble in ether, Soluble in hot ethanol.
Toxicological data
None yet
Ecological data
None yet
Molecular structure data
1. Molar refractive index: 70.80
2. Molar volume (cm3/mol): 193.6
3. Isotonic specific volume (90.2K ): 628.3
4. Surface tension (dyne/cm): 110.8
5. Dielectric constant:
6. Dipole moment (10-24cm3):
7. Polarizability: 28.06
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): -4.2
2. Number of hydrogen bond donors: 8
3. Number of hydrogen bond acceptors: 11
4. Number of rotatable chemical bonds��: 4
5. Number of tautomers: None
6. Topological molecule polar surface area 190
7. Number of heavy atoms: 23
8. Surface charge: 0
9. Complexity: 348
10. Number of isotope atoms: 0
11. Determine Number of atomic stereocenters: 10
12. Uncertain number of atomic stereocenters: 0
13. Determined number of chemical bond stereocenters: 0
14 .The number of uncertain chemical bond stereocenters: 0
15. The number of covalent bond units: 1
Properties and stability
1. Water loss above 130℃.
Storage method
Packaging in plastic bags coated with kraft paper bags or woven bags. Store in a cool, dry and ventilated place. Do not store together with toxic chemicals.
Synthesis method
1. It exists in free form in nature in mushrooms and other fungi, seaweed, shrimp, baker’s yeast and beer yeast. Preparation method: use starch as raw material, liquefy it, ferment it with heat-resistant α-amylase, saccharify it, and then use isomerizing amylase to get it.
2.Produced by fermentation method, the fermentation liquid is passed through anion exchange resin (amberlite CG 1201), and trehalose with a purity of 98.4% can be obtained.
Purpose
1. In medicine, it can be used in the stabilization of reagents and diagnostic drugs. In cosmetics, it can be used as a raw material for cosmetics production and has the effect of inhibiting dry skin and moisturizing. Trehalose can be used as a sweetener, taste improver and stabilizer in the formulation of lipsticks, lipsticks, etc. In the food industry, it can be used as a sweetener with non-reducing, moisturizing, anti-freezing and drying resistance properties.
2.Trehalose has good affinity and stability with membrane proteins. It can be used as a skin penetrating agent in external skin products to increase the absorption of nutrients by the skin. It is better to prepare liposomes when combined with phospholipids. It has excellent moisturizing properties and is effective in treating increased dandruff, dry and hot skin, and hardened cuticles caused by dry skin. Use 5% to 10% in creams, lotions, and ointments.
