2-Ethyl-2-methylsuccinimide Ethosuximide
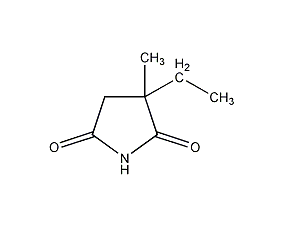

Structural formula
| Business number | 01LZ |
|---|---|
| Molecular formula | C7H11NO2 |
| Molecular weight | 141.17 |
| label |
ethosuximide, Zilangding, Chai Langding, 2-ethyl-2-methyl-succinimid, 3-Ethyl-3-methyl-2,5-pyrrolidinedione |
Numbering system
CAS number:77-67-8
MDL number:MFCD00072123
EINECS number:201-048-7
RTECS number:WN2800000
BRN number:None
PubChem number:24278423
Physical property data
1. Characteristics: white or slightly yellow waxy solid
2. Density (g/mL,25/4℃): Unsure
3. Relative vapor density (g/mL,AIR=1): Unsure
4. Melting point (ºC):64 -65℃
5. Boiling point (ºC,Normal pressure): Uncertain
6. Boiling point (ºC,5.2kPa): Unsure
7. Refractive index: Uncertain
8. Flash Point (ºC): Unsure
9. Specific optical rotation (º): Unsure
10. Autoignition point or ignition temperature (ºC): Unsure
11. Vapor pressure (kPa,25ºC): Unsure
12. Saturated vapor pressure (315.6
4. Surface Tension (dyne/cm):31.0
5. Polarizability(10-24cm3):14.26
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 1
5. Number of tautomers: 5
6. Topological molecule polar surface area 46.2
7. Number of heavy atoms: 10
8. Surface charge: 0
9. Complexity: 188
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 1
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
None yet
Storage method
This The product should be stored in a sealed container at room temperature.
Synthesis method
By 2-ethyl-2-Methyl succinic acid is obtained by cyclization with ammonia water . Add ammonia (20% ) Join2-ethyl-2-Methyl succinic acid, topHfor10Above. Heat to200-220℃, response20min. Cold to50℃The following, add chloroform. Filter, wash the filter cake with chloroform, discard the combined wash and filtrate, and separate the chloroform layer. Recover chloroform and distill under reduced pressure to collect 150 -160℃(2.13kPa) fraction, Get ethosuximide.
Purpose
Yes A newer class of anti-epileptic drugs used primarily for minor seizures. It has less toxicity and good effect. It is the first choice drug for minor attacks and is widely used. Ethosuximide can aggravate major seizures, so for patients with mixed major seizures, this product cannot be used alone and must be combined with benmabital or phenytoin.
yle=”FONT-SIZE: 9pt; FONT-FAMILY: 宋体; mso-ascii-font-family: ‘Times New Roman’; mso-hansi-font-family: ‘Times New Roman'”>for10Above. Heat to200-220℃, response20min. Cold to50℃The following, add chloroform. Filter, wash the filter cake with chloroform, discard the combined wash and filtrate, and separate the chloroform layer. Recover chloroform and distill under reduced pressure to collect 150 -160℃(2.13kPa) fraction, Get ethosuximide.
Purpose
Yes A newer class of anti-epileptic drugs used primarily for minor seizures. It has less toxicity and good effect. It is the first choice drug for minor attacks and is widely used. Ethosuximide can aggravate major seizures, so for patients with mixed major seizures, this product cannot be used alone and must be combined with benmabital or phenytoin.
