Dimethyl sulfide Dimethyl sulfide borane
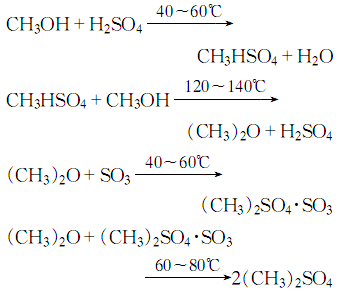
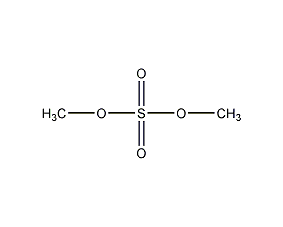
Structural formula
| Business number | 01M6 |
|---|---|
| Molecular formula | C2H6O4S |
| Molecular weight | 126.13 |
| label |
Methyl sulfate, dimethyl sulfate, dimethyl sulfate, Dimethoxysulfone, Dimethyl monosulfate, Methyl sulfate, Sulfuric acid dimethyl ester, Aliphatic carboxylic acids and their derivatives |
Numbering system
CAS number:77-78-1
MDL number:MFCD00008416
EINECS number:201-058-1
RTECS number:WS8225000
BRN number:635994
PubChem number:24893559
Physical property data
1. Properties: colorless or light yellow transparent liquid with a slight onion odor. [1]
2. pH value: <7 (1% solution) [2]
3. Melting point ( ℃): -31.8[3]
4. Boiling point (decomposition, ℃): 188[4]
5 .Relative density (water=1): 1.33 (20℃)[5]
6. Relative vapor density (air=1): 4.35[6]
7. Saturated vapor pressure (kPa): 2.00 (76°C) [7]
8. Critical pressure (MPa): 7.01 [8]
9. Octanol/water partition coefficient: 0.16[9]
10. Flash point (℃ ): 83 (OC); 83.3 (CC) [10]
11. Ignition temperature (℃): 188[11]
12.. The upper limit of explosion (%): 23.3[12]
13. The lower limit of explosion (%): 3.6[13]
14. Solubility: Slightly soluble in water, soluble in ethanol, ether, acetone, etc. [14]
Toxicological data
1. Human inhalation LCLo: 97ppm/10M. Rat oral LD50: 205mg/kg; inhalation LC50: 45mg/m3/4H. Mouse oral LC50: 140 mg/kg; inhalation LC50: 280 mg/m3. Dimethyl sulfate is highly toxic, its effects are similar to mustard gas, its acute toxicity is similar to phosgene, and is 15 times greater than chlorine. It has a strong irritating effect on the eyes and upper respiratory tract, and a strong corrosive effect on the skin. It can cause conjunctival congestion, edema, corneal epithelial shedding, partial necrosis of tracheal and bronchial epithelial cells, and puncture leading to mediastinal or subcutaneous emphysema. In addition, it can also damage the liver, kidneys and myocardium, and can cause burns, blisters and deep necrosis after skin contact. The mechanism of action is not yet fully understood. Most scholars believe that it is due to the methyl nature of the substance, which hydrolyzes into methanol and sulfuric acid in the body, causing toxic effects. This has been confirmed by animal experiments and the detection of methanol in the blood and internal organs of dead cases. Ghiringhelli believes that the local effects on the eyes and skin are partly due to sulfuric acid, while the systemic and nervous system effects, as well as pulmonary edema, are due to the toxic effects of the dimethyl sulfate molecule itself, which can oxidize certain important groups of methyl groups in the body. Caused by basalization. In addition to its corrosive effect on the skin, dimethyl sulfate may also cause contact allergic dermatitis. In recent years, foreign animal experiments have reported that acute dimethyl sulfate poisoningbsp;
1. In industry, the reaction of dimethyl ether and sulfur trioxide is mainly used to produce dimethyl sulfate. First, dimethyl ether is obtained by dehydrating methanol. Then heat 25% fuming sulfuric acid to evaporate the sulfur trioxide gas, and spray it with dimethyl sulfate to absorb it. The dimethyl sulfate that has absorbed sulfur trioxide reacts with dimethyl ether gas in the synthesis tower to generate dimethyl sulfate. The purity of industrial product dimethyl sulfate is >98%.
Raw material consumption quota: methanol 550kg/t, fuming sulfuric acid (25%) 3000kg/t.
![]()
Press an appropriate amount of oleum into the evaporation tank and heat it to gradually increase the temperature. When the temperature rises to 140°C, sulfur trioxide begins to be released. After the generation is completed, the remaining sulfuric acid in the evaporation tank is taken away, and the generated sulfur trioxide is passed into the esterification tank. Then add an appropriate amount of conversion agent into the etherification tank and heat it with steam to raise the temperature to 130~140℃. At the same time, methanol is introduced at a certain flow rate to generate dimethyl ether gas. After washing with sulfuric acid to remove water, it flows into the esterification tank. In the esterification tank, sulfur trioxide reacts with dimethyl ether to produce crude dimethyl sulfate, and the reaction temperature is controlled at 88 to 92°C. Send the crude dimethyl sulfate to the evaporator, steam out the dimethyl sulfate at 110-140°C and a vacuum of 94.7KPa, and then collect it in the refined ester storage tank after condensation. The distillation residue is neutralized by adding ammonia to generate ammonium sulfate, which is pressed into a storage tank as a by-product for recovery and used as fertilizer.
2.React methanol with sulfuric acid to generate methyl hydrogen sulfate, and then continue to react with methanol to generate dimethyl Ether gas reacts with sulfur trioxide to form dimethyl sulfate:
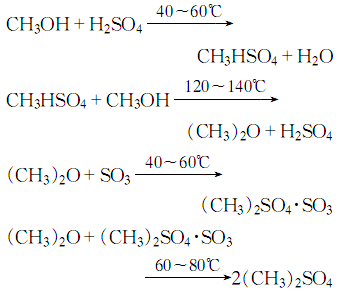
2. Preparation method:
Equipped with stirrer, thermometer, dripping liquid In the reaction bottle of the funnel, add 200g (1.71mol) of chlorosulfonic acid, cool to 0°C in an ice bath, and slowly add 54g (1.74mol) of methanol (2) dropwise while stirring. After the addition is complete, the reaction is continued to stir for 1 hour. Add anhydrous calcium chloride and dry. Distill under reduced pressure and collect the fraction at 138~140℃/2.66kPa to obtain 84~90g of dimethyl sulfate (1) with a yield of 80%~85%. [30]
Purpose
1. Dimethyl sulfate is a methylating agent widely used in organic synthesis in pesticides, dyes, medicines, perfume industries, etc. Used to make methyl ester, methyl ether, methylamine, etc. It is the raw material for dimethyl sulfoxide, caffeine, codeine, metamizole, aminopyridine, trimethoprim, vanillin and the pesticide acephate, etc. Used as solvent and alkylating agent for aromatic hydrocarbons.
2. Used as methylation reagent and solvent. Used in organic synthesis of medicines, pesticides, dyes, spices, etc.
3. Used in the manufacture of dyes and as a methylating agent for amines and alcohols. [29]
