Bretylium Tosylate Brettylium Tosylate
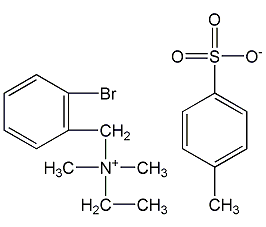

Structural formula
| Business number | 01C0 |
|---|---|
| Molecular formula | C18H24BrNO3S |
| Molecular weight | 414.36 |
| label |
Benzyl ammonium bromide, N-ethyl-N-o-bromobenzyl-N,N-dimethyl-ammonium p-toluenesulfonate |
Numbering system
CAS number:61-75-6
MDL number:MFCD00038731
EINECS number:200-516-8
RTECS number:BO9450000
BRN number:None
PubChem ID:None
Physical property data
1. Character:White crystalline powder
2. Density (g/mL,25/4℃): Unsure
3. Relative vapor density (g/mL,Air=1): Unsure
4. Melting point (ºC):97-99
5. Boiling point (ºC,Normal pressure): Uncertain
6. Boiling point (ºC, 5.2kPa): Unsure
7. Refractive index:Not sure
8. Flash Point (ºC): Unsure
9. Specific optical rotation (º): Unsure
10. Autoignition point or ignition temperature (ºC): Unsure
11. Vapor pressure (kPa,25ºC): Unsure
12. 17. Explosion limit (%,V/V): Unsure
18. Lower explosion limit (%,V/V): Unsure
19. Solubility:Easily soluble in water, alcohol, acetone, insoluble in benzene, ether, ethyl acetate, hexane
Toxicological data
None yet
Ecological data
None yet
Molecular structure data
None yet
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 3
4. Number of rotatable chemical bonds: 3
5. Number of tautomers: none
6. Topological molecule polar surface area 65.6
7. Number of heavy atoms: 24
8. Surface charge: 0
9. Complexity: 349
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 2
Properties and stability
None yet
Storage method
This product should be sealed and stored in a dry place.
Synthesis method
Using o-toluidine as raw material, denitrification through diazotization It is replaced with bromine to generate o-bromotoluene, which is brominated with bromine under light to generate o-bromobenzylmethane, and then aminated with dimethylamine under pressure to obtain o-bromobenzyldimethylamine, and finally combined with p–Addition of ethyl methanesulfonate to obtain the finished product.
Purpose
This product is an adrenergic blocker. It can improve the ventricular fibrillation threshold, so it can prevent and treat ventricular fibrillation, directly strengthen myocardial contractility, improve atrioventricular conduction, and is used for ventricular arrhythmias caused by various causes, such as frequent premature beats and paroxysmal ventricular arrhythmias. tachycardia, ventricular flutter and fibrillation, especially due to antimonials–Style’s syndrome is more effective. In addition, it also has a certain effect on arrhythmias caused by electrolyte disorders, acid-base imbalance, or drug poisoning caused by digitalis, quinidine and other drugs. A better anti-arrhythmic drug.
amily: Arial; mso-hansi-font-family: Arial; mso-bidi-font-family: Arial; mso-ansi-language: EN-US; mso-fareast-language: ZH-CN; mso-bidi-language: AR-SA”>Addition of ethyl methanesulfonate to obtain finished product.
Purpose
This product is an adrenergic blocker. It can improve the ventricular fibrillation threshold, so it can prevent and treat ventricular fibrillation, directly strengthen myocardial contractility, improve atrioventricular conduction, and is used for ventricular arrhythmias caused by various causes, such as frequent premature beats and paroxysmal ventricular arrhythmias. tachycardia, ventricular flutter and fibrillation, especially due to antimonials–Style’s syndrome is more effective. In addition, it also has a certain effect on arrhythmias caused by electrolyte disorders, acid-base imbalance, or drug poisoning caused by digitalis, quinidine and other drugs. A better anti-arrhythmic drug.
