Terephthaloyl Dichloride Terephthaloyl Dichloride
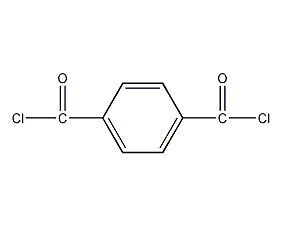

Structural formula
| Business number | 02HF |
|---|---|
| Molecular formula | C8H4Cl2O2 |
| Molecular weight | 203.02 |
| label |
1,4-Benzene dicarbonyl dichloride, Phthaloyl chloride, terephthaloyl chloride, terephthalyl dichloride, terephthaloyl chloride, Terephthalyl chloride, Terephthalic acid dichloride, p-Phthalodichloride, C6H4-1,4-(COCl)2, 1,4-Dichloroformylbenzene, p-Phenylenedicarbonyl dichloride, Aromatic carboxylic acids and their derivatives, Raw materials and intermediates used in ink |
Numbering system
CAS number:100-20-9
MDL number:MFCD00000693
EINECS number:202-829-5
RTECS number:WZ1797000
BRN number:607796
PubChem number:24888665
Physical property data
1. Properties: white solid or colorless needle-shaped crystals. [1]
2. Melting point (℃): 82~84[2]
3. Boiling point (℃) :266[3]
4. Relative vapor density (air=1): 7.0[4]
5. Saturated vapor pressure (kPa): 0.01 (38℃)[5]
6. Octanol/water partition coefficient: 0.880[6]
7. Flash point (℃): 180 (OC) [7]
8. Solubility: soluble in ether. [8]
Toxicological data
1. Acute toxicity:
Rat oral LD50: 2500mg/kg;
Rat inhalation LC50: 700mg/m3/4H;
Mouse oral LD50: 2140mg/kg;
Rabbit oral LD50: 950mg/kg;
2. Other multiple dose toxicity: Rat oral TDLo: 4168mg/kg/ 26W-I;
3. Reproductive toxicity:
Oral TDLo in male rats 26 weeks before mating: 4122mg/kgSEX/DURATION;
Before mating Oral TDLo of 26-week-old female rats: 4122mg/kgSEX/DURATION;
4. Acute toxicity[9] LD50: 2500mg/kg ( Orally administered to rats)
5. Irritation No data available
Ecological data
1. Ecotoxicity No data available
2. Biodegradability No data available
3. Non-biodegradability No information
Molecular structure data
1. Molar refractive index: 46.74
2. Molar volume (cm3/mol): 142.2
3. Isotonic specific volume (90.2K ): 374.9
4. Surface tension (dyne/cm): 48.2
5. Dielectric constant:
6. Dipole moment (10-24cm3):
7. Polarizability: 18.52
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 2
5. Number of tautomers: none
6. Topological molecule polar surface area 34.1
7. Number of heavy atoms: 12
8. Surface charge: 0
9. Complexity: 173
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Stability[10] Stable
2. Incompatible substances[11] Water, alcohols, strong oxidants, strong alkali
3. Conditions to avoid contact [12] Heat, humid air p>
4. Polymerization hazard[13] No polymerization
5. Decomposition products[14] sup> Phosgene, hydrogen chloride
Storage method
Storage Precautions[15] Store in a cool, dry and well-ventilated warehouse. Keep away from fire and heat sources. The packaging must be sealed and protected from moisture. They should be stored separately from oxidants, alkalis, alcohols, etc. and avoid mixed storage. Equipped with the appropriate variety and quantity of fire equipment. Suitable materials should be available in the storage area to contain spills.
Synthesis method
Carry out photocatalytic chlorination of para-xylene to prepare para-bis(trichloromethyl)benzene, then heat and reflux with terephthalic acid to perform acid chlorination reaction, then use solid sodium carbonate to make it alkaline, and filter out the chlorine After removing sodium, distill the liquid under reduced pressure and collect the 68-75°C (1.866kPa) fraction.
Purpose
1. It is a monomer of synthetic special fibers and can be used as reinforcing agent for aramid and nylon.
2. Used in the manufacture of resin dyes, pigments, medicines and pesticides, etc.
3. Used in organic synthesis. [16]
