Cyclohexylmethanol Cyclohexylmethanol
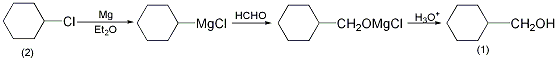
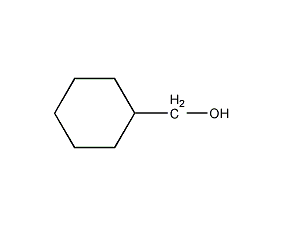
Structural formula
| Business number | 02J2 |
|---|---|
| Molecular formula | C7H14O |
| Molecular weight | 114.19 |
| label |
cyclohexylmethanol, Cyclohexanemethanol, cyclohexylmethane, cyclohexylmethanol, Hexahydrobenzyl alcohol, Hexahydrobenzyl alcohol, C6H11CH2OH, Benzyl Alcohol, Hexahydro-, Cyclohexylmethane, Cyclohexyl-Methano, Cyclohexylmethyl Alcohol, Hexahydro-Benzylalcoho, Methanol, Cyclohexyl-, Usaf Do-49, Usafdo-49 |
Numbering system
CAS number:100-49-2
MDL number:MFCD00001510
EINECS number:202-857-8
RTECS number:GV5075000
BRN number:773712
PubChem number:24879554
Physical property data
1. Properties: colorless liquid.
2. Density (g/mL, 20℃): 0.928
3. Relative vapor density (g/mL, air=1): 3.2
4. Melting point (ºC): -43
5. Boiling point (ºC, normal pressure): 183
6. Boiling point (ºC, 2.4KPa): 91
7. Refractive index: 1.4649
8. Flash point (ºC): 71
9. Relative density (20℃, 4℃): 0.9295
10. Refractive index at room temperature (n20): 1.4648
11. Refractive index at room temperature (n25): 1.4628
12. Liquid phase standard combustion heat (enthalpy) (kJ·mol-1): -43773
13. Liquid phase standard claimed heat (enthalpy) (kJ·mol-1): -378.1
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. The logarithmic value of the oil-water (octanol/water) partition coefficient: Undetermined
17. The upper limit of explosion (%, V/V): Undetermined
18. Lower explosion limit (%, V/V): Undetermined
19. Solubility: Soluble in alcohol and ether.
Toxicological data
Acute toxicity: mouse peritoneal cavity LD50: 250mg/kg;
Ecological data
This substance is slightly hazardous to water.
Molecular structure data
1. Molar refractive index: 33.89
2. Molar volume (cm3/mol): 125.0
3. Isotonic specific volume (90.2K): 301.3
4. Surface tension (dyne/cm): 33.7
5. Dielectric Constant:
6. Dipole moment (10-24cm3):
7. Polarizability: 13.43
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 1.9
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 1
5. Number of tautomers: none
6. Topological molecule polar surface area 20.2
7. Number of heavy atoms: 8
8. Surface charge: 0
9. Complexity: 55.4
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
Avoid contact with oxidants and acids.
Storage method
Store in a cool, ventilated warehouse. Keep away from fire and heat sources. They should be stored separately from oxidants and acidic substances, and avoid mixed storage. Keep sealed. Use explosion-proof lighting and ventilation facilities. It is prohibited to use mechanical equipment and tools that are prone to sparks. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
1. Chlorocyclohexane, absolute ether and magnesium chips are first used to prepare Grignard reagent, and then react with formaldehyde to generate cyclohexylmethanol.
2. Preparation method:
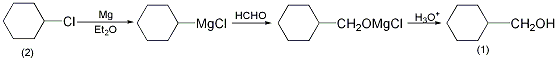
In a dry reaction bottle equipped with a stirrer, thermometer, reflux condenser (installed with a calcium chloride drying tube) and dropping funnel, add 26.7g (1.1mol) of magnesium chips and a small amount of crystallized iodine . In addition, 118.5g (1mol) of cyclohexyl chloride (2) was mixed with 450mL of diethyl ether dried with metallic sodium to prepare a solution. First add 100 mL of this solution and heat it in a water bath to initiate the reaction. When the reaction proceeds vigorously, remove the water bath and cool if necessary. And slowly add the remaining solution dropwise, and finish the addition in about 45 minutes. After the addition is completed, continue the reaction for 15 to 20 minutes. In a small Erlenmeyer flask, add 50g (1.67mol) of P2O5 dry paraformaldehyde, remove the dropping funnel from the above reaction bottle, and connect it to the Erlenmeyer flask with a rubber tube. With vigorous stirring, slowly add paraformaldehyde in batches. After reacting for 2 hours, cool the reaction mixture, pour it into 300g of crushed ice, and stir vigorously until it is completely decomposed. Slowly add 30% sulfuric acid to dissolve the generated magnesium hydroxide, and then evaporate the ether. The remaining water is steam distilled until no oil is evaporated. The leachate was saturated with sodium chloride, and an oily substance precipitated, which was extracted with diethyl ether. Dry over anhydrous potassium carbonate and evaporate the ether. Add 5g of fresh calcium oxide and heat in water bath for 30 minutes. Filter, distill under reduced pressure, and collect the fraction at 88-93°C/2.4kPa to obtain 50g of cyclohexylmethanol (1), with a yield of 44%. [1]
Purpose
Organic synthesis intermediates.
