Sec-Butyl alcohol sec-Butyl alcohol
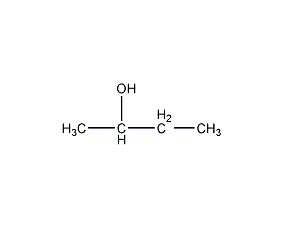

Structural formula
| Business number | 01NW |
|---|---|
| Molecular formula | C4H10O |
| Molecular weight | 74.12 |
| label |
2-butanol, Other butanol, Second butanol, 2-Butanol, sec-Butanol, Nitro spray paint flux, anti-emulsifier, dye dispersants, dehydrating agent, paint stripper, industrial detergent, alcohol compounds |
Numbering system
CAS number:78-92-2
MDL number:MFCD00004569
EINECS number:201-158-5
RTECS number:EO1750000
BRN number:773649
PubChem number:24857740
Physical property data
1. Properties: Colorless and transparent liquid with a wine-like smell. [1]
2. Melting point (℃): -114.7[2]
3. Boiling point (℃): 99.5[3]
4. Relative density (water = 1): 0.81[4]
5. Relative vapor Density (air=1): 2.6[5]
6. Saturated vapor pressure (kPa): 1.6 (20℃)[6]
7. Heat of combustion (kJ/mol): -2668.3[7]
8. Critical temperature (℃): 263[8]
9. Critical pressure (MPa): 4.202[9]
10. Octanol/water partition coefficient: 0.61 [10]
11. Flash point (℃): 23 (OC) [11]
12. Ignition temperature (℃ ): 406[12]
13. Explosion upper limit (%): 9.8[13]
14. Explosion lower limit (%): 1.7[14]
15. Solubility: soluble in water, miscible in ethanol, ether, and aromatic hydrocarbons. [15]
16. Viscosity (mPa·s, 20ºC): 4.210
17. Heat of evaporation (KJ/kg, b.p.): 562.6
18. Heat of formation (KJ/mol): -279.09
19. Specific heat capacity (KJ/(kg·K), 20ºC, constant pressure): 2.81
20. Solubility (%, water, 20ºC): 12.5
21. Volume expansion coefficient (K-1, 20ºC): 0.00097
22. Relative density (25℃, 4℃): 0.8026
23. Refractive index at room temperature (n25): 1.3949
24. Critical density (g· cm-3): 0.276
25. Critical volume (cm3·mol-1): 269 p>
26. Critical compression factor: 0.253
27. Eccentricity factor: 0.570
28. Lennard-Jones parameter (A): 13.86
29. Lennard-Jones parameter (K): 154.1
30. Solubility parameter (J·cm-3)0.5: 22.630
31.van der Waals area (cm2·mol-1): 7.620×109
32.van der Waals volume (cm3·mol-1): 52.390
33. Gas phase standard combustion heat (enthalpy) (kJ· mol-1): 2710.19
34. Gas phase standard claims heat (enthalpy) (kJ·mol-1): -293.01
35. Gas phase�Quasi entropy (J·mol-1·K-1): 359.53
36. Gas phase standard free energy of formation (kJ·mol-1): -167.9
37. Gas phase standard hot melt (J·mol-1·K-1): 112.14
38. Liquid phase standard combustion heat (enthalpy) (kJ·mol-1): -2660.44
39. Liquid phase standard claimed heat ( Enthalpy) (kJ·mol-1): -342.75
40. Liquid phase standard entropy (J·mol-1·K -1): 223.0
41. Liquid phase standard free energy of formation (kJ·mol-1): -177.19
42. Liquid phase standard hot melt (J·mol-1·K-1): 197.1
Toxicological data
1. Acute toxicity[16]
LD50: 2193mg/kg (rat oral); 4893mg/kg (rabbit oral ); >2000mg/kg (rat transdermal)
2. Irritation[17]
Rabbit Skin: 500mg (24h), mild irritation.
Rabbit eye: 100mg (24h), moderate irritation.
3. Others[18] Minimum toxic concentration for inhalation in rats (TCLo): 5000ppm/7h (gestation 1~19d), causing embryotoxicity .
Ecological data
1. Ecotoxicity[19]
LD50: 4300mg/L (24h) (goldfish)
IC50 : 312mg/L (72h) (algae)
2. Biodegradability[20]
Aerobic biodegradation (h): 24~168
Anaerobic biodegradation (h): 96~672
3.Abiodegradability[21]
Photooxidation half-life in water (h): 3100~1.00×106
Photooxidation half-life in air (h): 7.2 ~72
Molecular structure data
1. Molar refractive index: 22.07
2. Molar volume (cm3/mol): 92.4
3. Isotonic specific volume (90.2K ): 205.4
4. Surface tension (dyne/cm): 24.3
5. Polarizability (10-24cm3): 8.75
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 1
5. Number of tautomers: none
6. Topological molecule polar surface area 20.2
7. Number of heavy atoms: 5
8. Surface charge: 0
9. Complexity: 19.6
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 1
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. It has the chemical reaction characteristics of secondary alcohols. Dry sec-butyl alcohol is non-corrosive to metals. If moisture is present, especially at high temperatures, it can corrode aluminum.
2. Stability[22] Stable
3. Incompatible substances[23] Acids, acid chlorides, acid anhydrides, strong oxidants, halogens
4. Polymerization hazards [24] No polymerization
Storage method
Storage Precautions[25] Stored in a cool, ventilated warehouse. Keep away from fire and heat sources. The storage temperature should not exceed 37℃. Keep container tightly sealed. They should be stored separately from oxidants, acids, halogens, etc., and avoid mixed storage. Use explosion-proof lighting and ventilation facilities. It is prohibited to use mechanical equipment and tools that are prone to sparks. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
1. It is obtained by adsorbing butene in cracked petroleum or natural gas with sulfuric acid and then hydrolyzing it with water vapor.
2. It can be obtained by shrinking and dehydrating acetaldehyde to crotonaldehyde, which can be obtained by catalytic hydrogenation. Or it can be made from cereal starch as raw material through fermentation and separation.
3. It can be obtained by refining industrial product sec-butanol.
Purpose
1. Used as an intermediate in the production of methyl ethyl ketone, and used in the production of butyl acetate, sec-butyl acetate, etc. Used as anti-emulsifier, dye dispersant, dehydrating agent, paint remover, industrial detergent, etc. It is also used in the manufacture of plasticizers, mineral processing agents, herbicides, oil extractants, wetting agents, spices, etc. Since sec-butyl alcohol can increase the processability and ductility of paint, it can be used as a co-solvent for nitrocellulose spray paint and nitrocellulose paint thinner.
2. Butanol is mainly used to prepare edible flavors such as banana, cream, whiskey and cheese. 34 mg/kg in candies; 32 mg/kg in baked goods; 12 mg/kg in soft drinks; 7.0 mg/kg in cold drinks; 4.0 mg/kg in cream; and 1.0 mg/kg in alcohol.
3. Used as solvent and standard material for chromatographic analysis.
4. Used in the manufacture of methyl ethyl ketone, as a raw material for the synthesis of flavors, dyes, etc., and also as a solvent. [26]
