DL-Pantoyl Lacyone DL-Pantoyl Lacyone
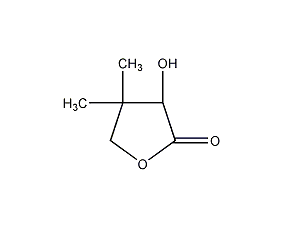

Structural formula
| Business number | 01Q1 |
|---|---|
| Molecular formula | C6H10O3 |
| Molecular weight | 130.14 |
| label |
DL-2-hydroxy-3,3-dimethyl-4-butyrolactone, DL-α-Hydroxy-β,β-dimethyl-γ-butyrolactone, Dihydro-3-hydroxy-4,4-dimethyl-2(3H)-furanone, DL-Pantolactone |
Numbering system
CAS number:79-50-5
MDL number:MFCD00064333
EINECS number:201-210-7
RTECS number:None
BRN number:80958
PubChem number:24879247
Physical property data
1. Characteristics: deliquescent columnar crystals.
2. Density (g/mL,25/4℃): Unsure
3. Relative vapor density (g/mL,AIR=1): Unsure
4. Melting point (ºC):80 ℃
5. Boiling point (ºC,Normal pressure):130℃( 2.4kPa)
6. Boiling point (ºC,5.2kPa): Unsure
7. Refractive index: Uncertain
8. Flash point (ºC): Unsure
9. Specific optical rotation (º): Unsure
10. Autoignition point or ignition temperature (ºC): Unsure
11. Vapor pressure (kPa,25ºC): Unsure
12.3. Isotonic specific volume (90.2K): 277.4
4. Surface Tension (dyne/cm):38.1
5. Polarizability(10-24cm3):12.32
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 0.5
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 3
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: 3
6. Topological molecule polar surface area 46.5
7. Number of heavy atoms: 9
8. Surface charge: 0
9. Complexity: 139
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 1
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
None yet
Storage method
This product should be sealed and stored in a cool place.
Synthesis method
Produced from isobutyraldehyde2,2-Dimethyl-3-After adding hydroxypropionaldehyde with sodium cyanide, it is obtained by hydrolysis, acidification and lactonization. 2,2- Dimethyl-3- Dissolve hydroxypropionaldehyde in water, put it into the reaction pot, and then add sodium cyanide aqueous solution and calcium chloride aqueous solution. Stir well and add 50% sulfuric acid solution. The reaction produces highly toxic hydrogen cyanide tail gas, which is absorbed by ferrous sulfate solution. At60-65℃ Reaction6hAfter that, heat up To80-85℃ Reaction3h, reduce stress Concentrate, add 95%95% -SIZE: 9pt; FONT-FAMILY: 宋体; mso-ascii-font-family: Arial; mso-hansi-font-family: Arial; mso-font-kerning: 0pt; mso-bidi-font-family: Arial”> Ethanol precipitates inorganic salts. Filtration, the filtrate is decompressed to recover ethanol and then fractionated to collect130-145 ℃(1.33-2.44 kPa) fraction, get DL-Panthenolactone.
Purpose
This product is an intermediate of pantothenic acid and is also used in other organic synthesis.
Arial; mso-font-kerning: 0pt”>-3-Hydroxypropionaldehyde is added with sodium cyanide, and then hydrolyzed, acidified, and lactonized to obtain it.2,2-Dimethyl -3-hydroxypropyl Dissolve the aldehyde in water, put it into the reaction pot, then add sodium cyanide aqueous solution and calcium chloride aqueous solution. Stir evenly and add 50%sulfuric acid solution. The reaction produces highly toxic hydrogen cyanide tail gas, which is absorbed by ferrous sulfate solution. At60-65℃ Reaction6hAfter that, heat up To80-85℃ Reaction3h, reduce stress Concentrate, add 95%95% -SIZE: 9pt; FONT-FAMILY: 宋体; mso-ascii-font-family: Arial; mso-hansi-font-family: Arial; mso-font-kerning: 0pt; mso-bidi-font-family: Arial”> Ethanol precipitates inorganic salts. Filtration, the filtrate is decompressed to recover ethanol and then fractionated to collect130-145 ℃(1.33-2.44 kPa) fraction, get DL-Panthenolactone.
Purpose
This product is an intermediate of pantothenic acid and is also used in other organic synthesis.
