4-Aminonaphthalene-1-sulfonic acid 4-Aminonaphthalene-1-sulfonic acid
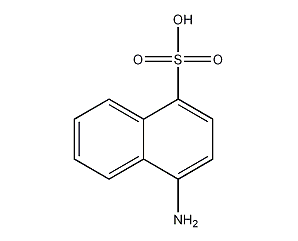

Structural formula
| Business number | 01V7 |
|---|---|
| Molecular formula | C10H9NO3S |
| Molecular weight | 223.25 |
| label |
1,4-Aminonaphthalenesulfonic acid, 4-amino-1-naphthalenesulfonic acid, Para-aminonaphthalene sulfonic acid, 1-Naphthylamine-4-sulfonic acid, 1-Naphthylamine-4-sulfonic acid, Naphthionic acid, H2NC10H6SO3H, Fluorescent dyes |
Numbering system
CAS number:84-86-6
MDL number:MFCD00004027
EINECS number:201-567-9
RTECS number:QK1270000
BRN number:1971299
PubChem number:24886126
Physical property data
1. Properties: White needle-like crystals. Sensitive to light and air. Decomposes when heated without melting.
2. Density (g/mL, 25/4℃): 1.673
3. Relative vapor density (g/mL, air=1): Undetermined
4. Melting point (ºC): ≥300℃ (decomposition)
5. Boiling point (ºC, normal pressure): Undetermined
6. Boiling point (ºC, 5.2kPa ): Not determined
7. Refractive index: Not determined
8. Flash point (ºC): Not determined
9. Specific rotation (º) : Undetermined
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (kPa, 25ºC): Undetermined
12. Saturated vapor pressure (kPa, 60ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) partition coefficient: Undetermined
17. Explosion Upper limit (%, V/V): Undetermined
18. Lower explosion limit (%, V/V): Undetermined
19. Solubility: Soluble in dilute hydrogen oxide Alkali or carbonic acid alkali solution has blue fluorescence. Solubility in water: 1 g of product dissolves in 3.45L at 10°C, 3.22L at 20°C, 1.69L at 50°C, and 0.438L at 100°C. It is extremely insoluble in ethanol and ether, almost insoluble in acetic acid and acetic anhydride, but can be dissolved when pyridine is added.
Toxicological data
1. Acute toxicity:
Rat caliber LD50: >7500mg/kg;
Mouse abdominal cavity LD50: 300mg/kg;
Ecological data
None yet
Molecular structure data
1. Molar refractive index: 58.27
2. ��� Mol volume (cm3/mol): 148.6
3. Isotonic specific volume (90.2K): 433.1
4. Surface tension (dyne/ cm): 72.1
5. Polarizability (10-24cm3): 23.10
Compute chemical data
1. Hydrophobic parameter calculation reference value (XlogP): 1.2
2. Number of hydrogen bond donors: 2
3. Number of hydrogen bond acceptors: 4
4. Number of rotatable chemical bonds: 1
5. Topological molecular polar surface area (TPSA): 80.4
6. Number of heavy atoms: 15
7. Surface charge: 0
8. Complexity: 322
9. Number of isotope atoms: 0
10. Determine the number of atomic stereocenters : 0
11. The number of uncertain atomic stereocenters: 0
12. The number of determined chemical bond stereocenters: 0
13. Uncertain chemical bond stereocenters Number of structural centers: 0
14. Number of covalent bond units: 1
Properties and stability
The sodium salt is easily soluble in water and is a large white monoclinic prism crystal with blue fluorescence.
Storage method
This product should be sealed and stored in a cool, dark place.
Synthesis method
Sulfonation of naphthylamine gives 4-amino-1-naphthalenesulfonic acid, which is neutralized to give the sodium salt. The sulfonation reaction is carried out in the solvent trichlorobenzene, the reaction temperature is 185-190°C, the vacuum degree is controlled at 2.67-5.33kPa, the insulation reaction is 3.5 hours, and the end point is checked by sampling. After passing the test, lower the temperature to 150°C, add alkaline water to neutralize, and control pH=8-9. At 70-80°C, add carbolic acid and alkali sulfide to the neutralized solution, raise the temperature to 90-95°C, then let it stand and separate into layers. The upper layer material liquid is concentrated, cooled, crystallized, and centrifuged to dryness to obtain the finished sodium salt. The yield is 83%. The lower trichlorobenzene is washed with water and recycled for reuse.
Purpose
Used in organic synthesis. Dye intermediates.
Dye intermediates. It is used to manufacture acid red A and B, acid red 3R, direct red 4B, direct red GB, direct red B, etc., and is used to produce intermediates such as 1-naphthol-4-sulfonic acid.
