Acetone Acetone
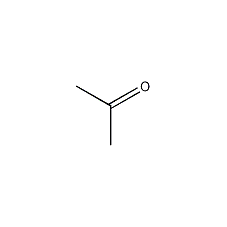

Structural formula
| Business number | 01EV |
|---|---|
| Molecular formula | C3H6O |
| Molecular weight | 58.08 |
| label |
2-acetone, Asitong, dimethyl ketone, acetone, Dimethylketone, Methyl acetyl, 2-Propanone, Dimethylketone, β-Ketopropane, Pyroacetic ether |
Numbering system
CAS number:67-64-1
MDL number:MFCD00008765
EINECS number:200-662-2
RTECS number:AL3150000
BRN number:635680
PubChem number:24883758
Physical property data
1. Properties: Colorless, transparent and flowing liquid, with aromatic odor and highly volatile. [1]
2. Melting point (℃): -95[2]
3. Boiling point (℃): 56.5[3]
4. Relative density (water = 1): 0.80[4]
5. Relative vapor Density (air=1): 2.00[5]
6. Saturated vapor pressure (kPa): 24 (20℃)[6]
7. Heat of combustion (kJ/mol): -1788.7[7]
8. Critical temperature (℃): 235.5[8]
9. Critical pressure (MPa): 4.72[9]
10. Octanol/water partition coefficient: -0.24[10]
11. Flash point (℃): -18 (CC); -9.4 (OC) [11]
12. Ignition temperature (℃): 465[12]
13. Explosion upper limit (%): 13.0[13]
14. Lower explosion limit (%): 2.2[14]
15. Solubility: miscible with water, miscible with ethanol, ether, chloroform, Most organic solvents such as oils and hydrocarbons. [15]
16. Refractive index (n20ºC): 1.3589
17. Refractive index (n25ºC): 1.3560
18 . Viscosity (mPa·s, 25ºC): 0.316
19. Specific rotation (º, c=3, in chloroform): +199.3
20. Heat of evaporation (KJ/ mol, 56.1ºC): 29.11
21. Heat of fusion (KJ/mol, 15ºC): 5.72
22. Heat of formation (KJ/mol, 25ºC, liquid): 246.98
23. Heat of combustion (KJ/mol, 25ºC, liquid): 1791.0
24. Specific heat capacity (KJ/(kg·K), 25ºC, constant pressure, gas): 1.28
25. Boiling point rising constant: 1.71
26. Conductivity (S/m, 25ºC): 5.8×10-8
27. Thermal conductivity (W/(m·K), room temperature): 1.9762
28. Volume expansion coefficient (K-1, 20ºC): 0.00144
29. Volume expansion coefficient (K-1, average at 0~100ºC): 0.00162
30. Critical density (g·cm-3): 0.273
31. Critical volume (cm3·mol-1): 213
32. Critical Compression factor: 0.237
33. Eccentricity factor: 0.306
34. Lennard-Jones parameter (A): 5.1864
35. Lennard-Jones parameter (K ): 148.07
36. Solubility parameter (J·cm-3)0.5: 19.774
37. van der Waals area (cm2·mol<suCalcium phosphate or potassium carbonate is dried and then distilled; it is also possible to use alkaline silver nitrate solution and acetone, filter, dry and then distill to remove organic impurities. To obtain high-purity acetone, react acetone with sodium bisulfite, filter out the resulting addition product, wash with ether, decompose with sodium carbonate solution, dry with anhydrous potassium carbonate and then distill. You can also react sodium iodide with acetone, filter out the crystals, and heat to decompose pure acetone. The specific operation is as follows:
① In 1000mL acetone, add 4g silver nitrate dissolved in 30mL water and 30mL 1mol/L sodium hydroxide solution. After stirring for 10 minutes, filter, dry with anhydrous calcium sulfate and then distill.
② Add powdered potassium permanganate to acetone in batches, and heat and reflux until the purple color does not disappear. Then add anhydrous calcium sulfate to dry, filter and fractionate. This method is suitable for refining large amounts of acetone.
③ Add 100g of powdered sodium iodide to 440g of boiling acetone, cool to -8°C with ice salt, and precipitate the adduct of sodium iodide and acetone (NaI·3C3H6O). After filtering, move it into a distillation bottle and heat it briefly to decompose pure acetone. Sodium iodide can be reused.
Acetone undergoes dehydration and condensation reaction under the action of acid or alkali. Therefore, it is difficult to obtain anhydrous materials. The above reaction can also occur when dehydrating with calcium chloride, phosphorus pentoxide, silica gel, alumina gel, sodium sulfate, and potassium carbonate, so it is better to use anhydrous calcium sulfate or magnesium sulfate as a desiccant.
(11) The main impurities in industrial acetone are water, aldehydes, alcohols and other organic compounds. You can first dehydrate with burned potassium carbonate, anhydrous calcium chloride or type 4A molecular sieve, then add 12% silver nitrate solution (34ml per liter of acetone) and then add 1mol/L sodium hydroxide solution (per liter of acetone). Add 30ml). Shake thoroughly and let it stand. After filtration, the filtrate obtained is dehydrated and dried with anhydrous calcium sulfate; if it contains reducing organic impurities, powdered potassium permanganate can be added to acetone in batches and heated while refluxing until the purple color is no longer generated. Until it fades again, evaporate the acetone and dehydrate it to dryness. Finally, the heating temperature is controlled to 65-70°C for distillation under normal pressure, and the 55-57°C fraction is collected, which is the finished product.
Purpose
1. Acetone is a representative low boiling point, quick-drying polar solvent. It has strong solubility and is soluble in water. In addition to being used as a solvent for coatings, varnishes, nitrocellulose spray paints, etc., it is also used as a solvent and paint stripper in the manufacture of cellulose, cellulose acetate, photographic film, etc. Acetone can extract various vitamins and hormones and dewax petroleum. Acetone is also an important chemical raw material used in the manufacture of acetic anhydride, methyl methacrylate, bisphenol A, isopropylene acetone, methyl isobutyl ketone, hexylene glycol, chloroform, iodoform, epoxy resin, and vitamin C. wait. And used as extraction agent, diluent, etc.
2. Used to prepare organic glass monomer, bisphenol A, diacetone alcohol, hexylene glycol, methyl isobutyl ketone, methyl isobutyl carbinol, phorone, isophorone Ketone, chloroform, iodoform and other important organic chemical raw materials. It is used as an excellent solvent in coatings, acetate fiber spinning process, acetylene storage in cylinders, dewaxing in the oil refining industry, etc. In the pharmaceutical industry, it is one of the raw materials for vitamin C and the anesthetic sophora, and is also used as an extraction agent in the production of various vitamins and hormones. In the pesticide industry, acetone is one of the raw materials for the synthesis of allylpyrethroids.
3. Used as analytical reagents, such as solvents. Used as chromatography derivative reagent and liquid chromatography eluent.
4. Used in the electronics industry, often used as cleaning and degreasing agents.
5. Commonly used as a solvent for vinyl resin, acrylic resin, alkyd paint, cellulose acetate and various adhesives. It is also widely used in the manufacture of cellulose acetate, film, film and plastics, and is also a raw material for the production of methyl methacrylate, methyl isobutyl ketone, bisphenol A, acetic anhydride, ketene and furan resin.
6. Can be used as diluent, detergent and extraction agent for vitamins and hormones.
7. It is a basic organic raw material and a low boiling point solvent. [27]
