Chloroform Chloroform
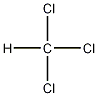
Structural formula
| Business number | 01EW |
|---|---|
| Molecular formula | CHCl3 |
| Molecular weight | 119.38 |
| label |
chloroform, Trichloromethane, Formyl trichloride, Methenyl chloride, Aliphatic halogenated derivatives |
Numbering system
CAS number:67-66-3
MDL number:MFCD00000826
EINECS number:200-663-8
RTECS number:FS9100000
BRN number:1731042
PubChem number:24892487
Physical property data
1. Characteristics: colorless, transparent and heavy liquid, highly volatile, with a special odor. [1]
2. Melting point (℃): -63.5[2]
3. Boiling point (℃): 61.3[3]
4. Relative density (water = 1): 1.5[4]
5. Relative vapor Density (air=1): 4.12[5]
6. Saturated vapor pressure (kPa): 21.2 (20℃)[6]
7. Critical temperature (℃): 263.4[7]
8. Critical pressure (MPa): 5.47[8]
9. Octanol/water partition coefficient: 1.97[9]
10. Solubility: insoluble in water, miscible in ethanol and ether , benzene, acetone, carbon disulfide, carbon tetrachloride. [10]
11. Viscosity (mPa·s, 20ºC): 0.563
12. Relative density (25℃, 4℃): 1.4797
13. Refractive index at room temperature (n20): 1.4459
14. Heat of evaporation (KJ/mol, b.p.): 247.0
15. Heat of fusion (KJ/mol): 9.55
16. Heat of formation (KJ/mol, 25ºC, liquid): 134.56
17. Heat of combustion (KJ/mol, 25ºC, liquid): 402.23
18. Specific heat capacity (KJ/(kg·K), 20ºC): 1.189
19. Boiling point rising constant: 3.88
20. Electrical conductivity (S/m, 25ºC): <1×10-10
21. Thermal conductivity (W/(m·K), 20ºC): 0.12997
22. Volume expansion coefficient (K-1): 0.001399
23. Critical density (g·cm-3 ): 0.491
24. Critical volume (cm3·mol-1): 243
25. Critical compression factor : 0.2691
26. Eccentricity factor: 0.213
27. Lennard-Jones parameter (A): 5.2751
28. Lennard-Jones parameter (K): 414.03
29. Solubility parameter (J·cm-3)0.5: 19.028
30. van der Waals area (cm2·mol-1): 7.660×109
31. van der Waals volume (cm3·mol-1): 43.500
32. Gas phase standard claims heat (enthalpy) (kJ·mol-1 ): -102.9
33. Gas phase standard entropy (J·mol-1·K-1): 295.61
34. Gas phase standard free energy of formation (kJ·mol-1): -70.1
35. Gas phase standard hot melt (J·mol-1 ·K-1): 65.38
36. Liquid phase standard claims heat (enthalpy) (kJ·mol-1): -134.31
37. Liquid phase standard entropy (J·mol-1·K-1): 202.9
38. Liquid phase standard Free energy of formation (kJ·mol-1): -73.93
39. Liquid phase standard hot melt (J·mol-1·K-1): 114.2
31/201008311511056348.gif” width=”168″ height=”20″ alt=”” />
3. First, wash the industrial chloroform with water until it is neutral, let it stand, and discard the upper layer of water. . Then mix it with industrial concentrated sulfuric acid (98%) at a ratio of 10:1, stir and wash until the pickling liquid turns light brown. Wash thoroughly each time, and let it stand for layering to remove the acid. After pickling, use chloroform for 15 % sodium carbonate solution in a ratio of 10:1, neutralize and remove acid until qualified, collect the lower layer of chloroform after standing. Finally, add a small amount of industrial anhydrous potassium carbonate for distillation, and collect the middle distillate (61~62°C), that is is the finished product.
4. Methanol method Methanol method is also called methanol hydrochlorination/chlorination method. The production of chloroform from methanol is carried out in two steps. First, the catalytic hydrochlorination of methanol is used to produce methyl chloride, and then Thermal chlorination of monochloromethane to produce chloroform. The industrial unit is divided into four processes: hydrochlorination, chlorination, distillation, and vaporization.
1) The hydrochlorination process uses methanol and hydrogen chloride as raw materials. There are two methods of methyl chloride: liquid phase catalysis and solid phase catalysis. The liquid phase catalysis method is under the action of a 75% to 80% zinc chloride aqueous solution catalyst at a pressure of 0.05MPa and a temperature of 140 to 145°C. The following reaction occurs between methanol and hydrogen chloride: 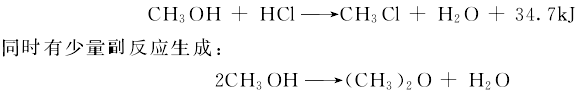
Monochlorine is generated in the reaction gas In addition to methane, water and dimethyl ether, there are also unreacted methanol and hydrogen chloride. This process uses a combination of condensation and absorption to separate the water generated by the reaction and unreacted methanol, hydrogen chloride and methyl chloride gas, and then use The alkali neutralizes the remaining hydrogen chloride. Then, the reaction gas is compressed, cooled and dried to remove moisture. Finally, it is sent to the chlorination process as raw material.
2) Chlorination process: Chlorine gas is mixed with a gas at 400~450℃ Methyl chloride undergoes the following reaction: 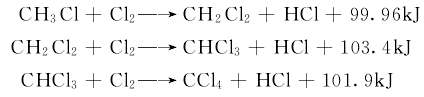
Change the ratio of chlorine to methyl chloride , which can adjust the distribution of chloride in the product. Industrial equipment achieves the main production of chloroform by controlling the chlorine ratio and recycling low chloride.
3) Distillation process monochloromethane chlorination Afterwards, the crude chlorinated liquid is obtained by condensation, and then processed using a 4-tower process. In the initial distillation tower, low boiling matter (hydrogen chloride, monochloromethane, dichloromethane) is removed. In the trichloride distillation tower, tetrachlorine is removed High boiling matter such as carbon dioxide is removed, and the chloroform product is obtained from the top of the tower. In the intermediate distillation tower and the tetrachlorine distillation tower, high boiling matter such as chloroform and tetrachlorethylene are removed respectively, and in the tetrachloride distillation tower The carbon tetrachloride product is obtained.
4) Vaporization process The purpose of this process is to heat and vaporize the liquid chlorine and then send it to the chlorination process as a raw material for the chlorination reaction.
5. It is prepared by the reduction of carbon tetrachloride, and can also be obtained industrially by the reaction of ethanol and hypochlorite.
Purpose
1. Mainly used in the production of Freon (F-21, F-22, F-23), dyes and drugs. In medicine, it is used as an extractant for anesthetics and natural or fermented drugs. It can also be used as a solvent and extractant for spices, oils, resins, and rubber. It can be mixed with carbon tetrachloride to make a non-freezing fireproof liquid. Fumigants can also be prepared and used as intermediates for insecticides and fungicides.
2. Used as analytical reagents, such as solvents and standard materials for chromatographic analysis. Also used in organic synthesis.
3. Used in the electronics industry, often used as cleaning and degreasing agents.
4. Used in organic synthesis, as solvents and anesthetics, etc. [27]
