1-Pentene 1-Pentene
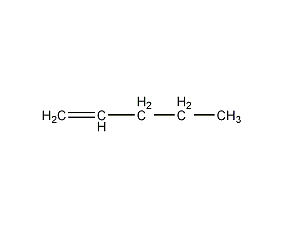

Structural formula
| Business number | 02ZG |
|---|---|
| Molecular formula | C5H10 |
| Molecular weight | 70 |
| label |
propylethylene, α-n-Amylene |
Numbering system
CAS number:109-67-1
MDL number:MFCD00003567
EINECS number:203-694-5
RTECS number:SB2179000
BRN number:1731629
PubChem number:24887095
Physical property data
1. Properties: colorless liquid with foul odor. [1]
2. Melting point (℃): -165.2[2]
3. Boiling point (℃): 29.9~30.1[3]
4. Relative density (water=1): 0.64[4]
5. Relative vapor density (air=1): 2.42[5]
6. Saturated vapor pressure (kPa): 70.7 (20℃)[6]
7. Heat of combustion (kJ/mol): -3347.2[7]
8. Critical temperature (℃): 201[ 8]
9. Critical pressure (MPa): 3.56[9]
10. Octanol/water partition coefficient: 2.66[10]
11. Flash point (℃): -28[11]
12. Ignition temperature (℃) ): 275[12]
13. Explosion upper limit (%): 8.7[13]
14. Explosion lower limit (%): 1.4[14]
15. Solubility: Insoluble in water, miscible in ethanol, ether, soluble in benzene, etc. [15]
16. Critical volume (cm3·mol-1): 298.4
17. Critical density (g·cm-3): 0.235
18. Critical compression factor: 0.275
19. Eccentricity factor: 0.233
20. Solubility parameter (J·cm-3)0.5: 14.599
21. Lennard-Jones parameter (A ): 9.122
22. Lennard-Jones parameter (K): 189.1
23. van der Waals area (cm2·mol– 1): 7.760×109
24. van der Waals volume (cm3·mol-1): 54.540
25. Gas phase standard combustion heat (enthalpy) (kJ·mol-1): -3375.19
26. Gas phase standard claim Heat (enthalpy) (kJ·mol-1): -21.51
27. Gas phase standard entropy (J·mol-1·K-1): 345.81
28. Gas phase standard free energy of formation (kJ·mol-1): 78.61
29. Gas phase Standard hot melt (J·mol-1·K-1): 106.44
30. Liquid phase standard combustion heat (enthalpy) (kJ· mol-1): -3349.72
31. Liquid phase standard claims heat (enthalpy) (kJ·mol-1): -46.97
32. Liquid phase standard entropy (J·mol-1·K-1): 262.60
33. Liquid phase standard Free energy of formation (kJ·mol-1): 77.94
34. Liquid phase standard hot melt (J·mol-1·K-1): 154.87
35. Gas phase standard hot melt (J·mol-1·K-1): 105.35
36. Liquid phase standard hot melt (J·mol-1·K-1): 163.6
Toxicological data
1. Acute toxicity[16]
LC50: 175000mg/m3 (rat inhalation, 4h ); 180000mg/m3 (mouse inhalation, 2h)
2. Irritation No information available
3.Subacute and chronic poisoning��[17] Rats inhaled pentene 0.01g/m3 day and night for 2.5 months, first excited and then inhibited, conditioned reflex disorder, serum Cholinesterase activity is reduced, porphyrins are increased in urine, and pulmonary bronchitis and interstitial inflammation are also seen.
Ecological data
1. Ecotoxicity No data available
2. Biodegradability No data available
3 .Non-biodegradability[18] In the air, when the concentration of hydroxyl radicals is 5.00×105/cm3 When, the degradation half-life is 12h (theoretical).
4. Other harmful effects[19] This substance may be harmful to the environment, special attention should be paid to surface water , soil, air and drinking water pollution.
Molecular structure data
1. Molar refractive index: 24.93
2. Molar volume (cm3/mol): 106.1
3. Isotonic specific volume (90.2K ): 220.0
4. Surface tension (dyne/cm): 18.4
5. Dielectric constant (F/m): 2.03
6. Polar Chemical rate (10-24cm3): 9.98
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 2.4
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 0
4. Number of rotatable chemical bonds: 2
5. Number of tautomers:
6. Topological molecular polar surface area (TPSA): 0
7. Number of heavy atoms: 5
8. Surface charge: 0
9. Complexity: 21.2
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters Number: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Stability[20] Stable
2. Incompatible substances[21] Strong oxidants, acids, halogenated hydrocarbons, halogens, etc.
3. Conditions to avoid contact[22] Heating
4. Aggregation hazards[23] Aggregation
Storage method
Storage Precautions[24] Store in a cool, ventilated warehouse. Keep away from fire and heat sources. The storage temperature should not exceed 29°C. The packaging must be sealed and must not come into contact with air. should be kept away from oxidizer, do not store together. It should not be stored in large quantities or for long periods of time. Use explosion-proof lighting and ventilation facilities. It is prohibited to use mechanical equipment and tools that are prone to sparks. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
1. Its preparation method is to obtain it by fractional distillation in petroleum cracked gas C5.
2. Prepared from allyl bromide and ethyl magnesium bromide in diethyl ether or propyl ether.
Purpose
Used in organic synthesis and preparation of isoprene, and also used as an additive for high-octane gasoline. [25]
