1-Butanethiol 1-Butanethiol
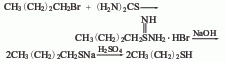
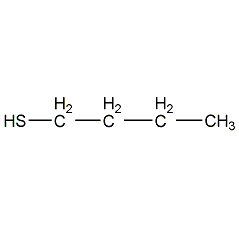
Structural formula
| Business number | 02ZT |
|---|---|
| Molecular formula | C4H10S |
| Molecular weight | 90.19 |
| label |
n-Butanethiol, 1-Thiobutanol, n-Butylmercaptan, Butylthiol, 1-Mercaptobutane, food additives, flavor enhancer, Sulfur compound solvents, alcohol solvent |
Numbering system
CAS number:109-79-5
MDL number:MFCD00004905
EINECS number:203-705-3
RTECS number:EK6300000
BRN number:1730908
PubChem number:24847120
Physical property data
1. Properties: colorless liquid with foul odor. [1]
2. Melting point (℃): -115.7[2]
3. Boiling point (℃): 97.2~101.7[3]
4. Relative density (water=1): 0.84[4]
5. Relative vapor density (air=1): 3.1[5]
6. Saturated vapor pressure (kPa): 6.07 (25℃)[6]
7. Heat of combustion (kJ/mol): -3481.7[7]
8. Critical pressure (MPa): 3.94[ 8]
9. Octanol/water partition coefficient: 2.28[9]
10. Flash point (℃): 2 ( CC)[10]
11. Ignition temperature (℃): 225[11]
12. Solubility : Slightly soluble in water, easily soluble in ethanol, ether, etc. [12]
Toxicological data
1. Acute toxicity[13]
LD50: 1500mg/kg (rat oral)
LC50 : 4020ppm (rat inhalation, 4h); 2500ppm (mice inhalation, 4h)
2. Irritation [14] Rabbit eye : 83mg, causes irritation.
Ecological data
1. Ecotoxicity[15] LC50: 5.5mg/L (48h) (bluegill sunfish)
2 .Biodegradability No data yet
3. Non-biodegradability [16] In the air, when the concentration of hydroxyl radicals is At 5.00×105 pieces/cm3, the degradation half-life is 9h (theoretical).
Molecular structure data
1. Molar refractive index: 28.47
2. Molar volume (cm3/mol): 108.6
3. Isotonic specific volume (90.2K ): 243.1
4. Surface tension (dyne/cm): 25.1
5. Polarizability (10-24cm3): 11.28
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 2
5. Number of tautomers: none
6. Topological molecule polar surface area 1
7. Number of heavy atoms: 5
8. Surface charge: 0
9.���Hurility: 13.1
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain atomic stereocenter Number of stereocenters: 0
13. Determine the number of stereocenters of chemical bonds: 0
14. Uncertain number of stereocenters of chemical bonds: 0
15. Total Number of price key units: 1
Properties and stability
1. It is easy to cause combustion when exposed to high heat, open flames and strong oxidants, and will decompose when heated to release toxic gases.
2. Stability[17] Stable
3. Incompatible substances[18] Alkalis, strong oxidants, alkali metals
4. Conditions to avoid contact [19] Heat
5. Polymerization hazard[20] No polymerization
6. Decomposition products[21] Hydrogen sulfide
Storage method
1. Storage precautions [22] Store in a cool, ventilated warehouse. Keep away from fire and heat sources. The storage temperature should not exceed 37°C. Keep container tightly sealed. They should be stored separately from oxidants, alkalis, and alkali metals, and avoid mixed storage. Use explosion-proof lighting and ventilation facilities. It is prohibited to use mechanical equipment and tools that are prone to sparks. The storage area should be equipped with emergency release equipment and suitable containment materials.
2. This product is packed in glass bottles with wooden boxes or calcium plastic boxes reinforced with padding or iron barrels. Store in a cool, dry and ventilated warehouse, away from fire and heat sources, and avoid direct sunlight. Store and transport in isolation from oxidants, acids and water-containing items.
Synthesis method
Originated from the reaction of butyl bromide and thiourea. Thiourea and butyl bromide were heated and refluxed in methanol solvent for 3 hours. After methanol was recovered, dilute alkali solution was added and reflux continued for 6 hours. Cool and slowly add 1:2 dilute sulfuric acid to pH 2. With the addition of dilute sulfuric acid, butanethiol will evaporate. After adding sulfuric acid, steam distillation until no oil beads are distilled. Separate the oil layer, dry it with anhydrous calcium chloride, fractionate and collect the 97-98°C fraction as the finished product.
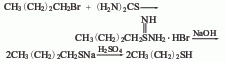
Purpose
Used as solvent and organic synthesis intermediate. [23]
