Acetaldehyde Acetaldehyde

Structural formula
| Business number | 01HX |
|---|---|
| Molecular formula | C2H4O |
| Molecular weight | 44.05 |
| label |
anhydrous acetaldehyde, Acetaldehyde, Methanecarbaldehyde, Ethanal, Acetic aldehyde, Aldehyde solvents, aliphatic compounds |
Numbering system
CAS number:75-07-0
MDL number:MFCD00006991
EINECS number:200-836-8
RTECS number:AB1925000
BRN number:505984
PubChem number:24844966
Physical property data
1. Properties: Colorless liquid with a strong irritating odor. [1]
2. Melting point (℃): -123.5[2]
3. Boiling point (℃): 20.8[3]
4. Relative density (water=1): 0.788 (16℃)[4]
5. Relative vapor density (air = 1): 1.52[5]
6. Saturated vapor pressure (kPa): 98.64 (20℃)[6]
7. Heat of combustion (kJ/mol): -1166.37[7]
8. Critical temperature (℃): 188[8]
9. Critical pressure (MPa): 6.4[9]
10. Octanol/water partition coefficient: 0.43[10]
11. Flash point (℃): -39 (CC); -40 (OC) [11]
12. Ignition temperature (℃): 175[12]
13. Explosion upper limit (%): 57[13]
14. Lower explosion limit (%): 4.0[14]
15. Solubility: soluble in water, miscible in ethanol, ether, Benzene, gasoline, toluene, xylene, etc. [15]
16. Critical density (g·cm-3): 0.286
17. Critical volume (cm 3·mol-1): 154
18.Lennard-Jones parameter (A): 11.98
19.Lennard -Jones parameter (K): 141.3
20. Solubility parameter (J·cm-3)0.5: 19.819
21.van der Waals area (cm2·mol-1): 4.490×109
22. van der Waals volume (cm3·mol-1): 28.810
23. Liquid phase standard heat of combustion (enthalpy) (kJ·mol-1): -1165.79
24. Liquid phase standard claims heat (enthalpy) (kJ·mol-1): -192.88
25. Liquid phase standard entropy (J·mol-1·K-1): 117.3
26. Liquid phase standard hot melt (J·mol-1·K-1): 89.05
27. Gas phase standard combustion heat (enthalpy) (kJ·mol -1): -1192.48
28. Gas phase standard claims heat (enthalpy) (kJ·mol-1): -166.19
29. Gas phase standard entropy (J·mol-1·K-1): 263.95
30. Gas phase standard formation free energy (kJ·mol -1): -133.0
31. Gas phase standard hot melt (J·mol-1·K-1 ): 55.32
Toxicological data
1. Acute toxicity[16]
LD50: 661mg/kg (rat oral)
LC50 : 13300ppm (rat inhalation, 4h)
2. Irritation/day_100907/201009071010236833.gif” alt=”” />
3. Acetylene direct hydration method Acetylene and water are directly hydrated under the action of mercury catalyst or non-mercury catalyst to obtain acetaldehyde. Due to the problem of mercury harm , has been gradually replaced by other methods.
![]()
4. Alcohol dehydrogenation method: Under the action of a copper catalyst added with cobalt, chromium, zinc or other compounds, alcohol is dehydrogenated to produce acetaldehyde.
5. Saturated hydrocarbon oxidation method.
![]()
Raw material consumption quota: acetylene hydration law Each ton of product consumes 610kg of 99% acetylene; the ethanol oxidation method consumes 1200kg of 95% ethanol; the ethylene oxidation method (one-step method) consumes 710kg of 99% ethylene and 300 cubic meters of oxygen (99%). Commercially available industrial products acetaldehyde, ethylene method B The purity of aldehyde is 99.7%, and the purity of ethanol method is 98%.
6. Preparation method:
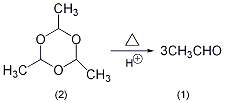
In the reaction bottle equipped with a fractionation device (the receiving bottle is cooled with an ice bath to reduce the volatilization loss of acetaldehyde), add paraacetaldehyde ( 2) 50g, 0.5 mol of concentrated sulfuric acid, a few grains of zeolite. Heat slowly, taking care not to make the temperature at the top of the fractionation column too high. The reaction should proceed slowly, because acetaldehyde and paraacetaldehyde can form an azeotrope, bp42 ℃ (the molar ratio is acetaldehyde: paraacetaldehyde = 53.4:46.6). Most of the acetaldehyde (1) is evaporated at 21 ~ 25 ℃. Stop distillation when there is approximately 10 mL left in the reaction bottle (if it is distilled to dryness) Risk of explosion). The acetaldehyde prepared in this way can meet most uses. If higher purity acetaldehyde is required, fractionation can be carried out again and the fraction at 21°C can be collected. [32]
7. Preparation method:
![]()
In a reaction bottle equipped with a ventilation tube, a dropping funnel, and a distillation device (the receiving bottle can be filled with two gas washing bottles connected in sequence, filled with ether, and cooled with an ice-salt bath), add 135 mL (about 2 mol) of ethanol (2) ), add 1/3 volume of dilute sulfuric acid composed of 150mL concentrated sulfuric acid and 250mL water. Pour in carbon dioxide gas and heat to boiling. After diluting another 2/3 volume of sulfuric acid with 100 mL of water, add 200 g of sodium dichromate, stir to dissolve, and drop this solution into the reactant. The reaction is exothermic. The generated acetaldehyde is continuously taken out by the carbon dioxide gas flow, and the acetaldehyde is dissolved in ether. Complete the dripping in about 30 minutes, and then continue to pass carbon dioxide for 10 minutes. A solution of acetaldehyde in diethyl ether was obtained. Acetaldehyde cannot be distilled from its ether solution. To obtain pure acetaldehyde, proceed as follows: cool the acetaldehyde ether solution in an ice-water bath, slowly pass in dry nitrogen, and shake continuously. During this period, acetaldehyde amine crystals precipitate until the precipitation is complete ①. Filter with suction, wash with anhydrous ether, and dry to obtain 55g of acetaldehydeamine (3). In a reaction bottle equipped with a ventilation tube, a dropping funnel, and a distillation device (the receiving bottle is cooled with an ice-salt bath), add compound (3), dissolve it in 50 mL of water, add carbon dioxide gas, heat in the water bath, and dropwise add 60 mL of concentrated The acetaldehyde generated from the dilute sulfuric acid mixed with sulfuric acid and 80 mL of water is continuously evaporated, and the dripping is completed in about 30 minutes. The boiling point of acetaldehyde is 21°C. Note: ① Take a small amount of transparent liquid and add nitrogen until it is saturated. If no precipitation precipitates, it means the reaction is over. [33]
Purpose
1. Used to prepare standard solutions, reducing agents, and bactericides when measuring aldehydes using colorimetric methods. Manufacture of paraacetaldehyde, acetic acid, butanol and pentaerythritol. Organic synthesis intermediates and solvents.
2. Industrially used to produce paraformaldehyde, acetic acid, ethyl acetate, pentaerythritol, plastics, synthetic rubber, synthetic resin, etc. It can also be used for spectrophotometric determination of aldehydes.
3. Reducing agent, bactericide, used to prepare standard solution when measuring aldehyde by colorimetric method. Industrially used to make polyacetaldehyde, acetic acid, synthetic rubber, etc.
4. Used to make acetic acid, acetic anhydride and synthetic resin. [31]
