Acetone oxime Acetone oxime
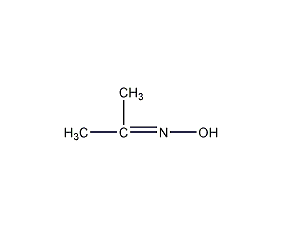

Structural formula
| Business number | 03KK |
|---|---|
| Molecular formula | C3H7NO |
| Molecular weight | 73.10 |
| label |
2-acetone oxime, dimethyl ketoxime, β-isonitrosopropane, 2-Propanonoxime, beta-Isonitrosopropane, aliphatic compounds |
Numbering system
CAS number:127-06-0
MDL number:MFCD00002118
EINECS number:204-820-1
RTECS number:AL6825000
BRN number:1560146
PubChem ID:None
Physical property data
1. Properties: White needle-like crystal
2. Relative density (62℃): 0.9113
3. Relative vapor density (g/mL, air=1): Undetermined
4. Melting point (ºC): 61
5. Boiling point: 136ºC, 134.8ºC (97kPa), 61ºC (2.67kPa)
6. Boiling point (ºC, 5.2kPa): Not determined
7. Refractive index: 1.1156
8. Flash point (ºC): Not determined
9. Ratio Optical rotation (º): Undetermined
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (kPa, 25ºC): Undetermined
p>
12. Saturated vapor pressure (kPa, 60ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC ): Undetermined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) partition coefficient: Undetermined
17. Explosion upper limit (%, V/V): Undetermined
18. Explosion lower limit (%, V/V): Undetermined
19. Solubility: soluble Miscible in ethanol, acetone, ether, chloroform and petroleum, soluble in water.
Toxicological data
1. Acute toxicity data:
Rat oral LD: >500 mg/kg
Mouse abdominal LC50: 4mg/kg
Small Rat route unknown: >3mg/kg
Ecological data
None
Molecular structure data
1. Molar refractive index: 19.90
2. Molar volume (cm3/mol): 80.2
3. Isotonic specific volume (90.2K ): 180.5
4. Surface tension (dyne/cm): 25.6
5. Polarizability (10-24cm3): 7.88
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 1
3. Hydrogen bondNumber of isomers: 2
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: 3
6. Topological molecular poles Surface area: 32.6
7. Number of heavy atoms: 5
8. Surface charge: 0
9. Complexity: 44.9
10. Number of isotope atoms: 0
11. Number of determined atomic stereocenters: 0
12. Number of uncertain atomic stereocenters: 0
13 .Determined number of stereocenters of chemical bonds: 0
14. Uncertain number of stereocenters of chemical bonds: 0
15. Number of covalent bond units: 1
Properties and stability
1. White prismatic, needle-like crystals or powder. It has an odor similar to chloral hydrate. Sensitive to moisture. Evaporates quickly in the air. Neutral reaction. Easily soluble in water, ethanol, ether and petroleum ether. Easily hydrolyzed in dilute acid. Relative density 0.9113. Melting point 60℃. Boiling point 134.8℃ (97.06kPa). Refractive index 1.4156. The median lethal dose (mice, abdominal cavity) is 4000mg/kg
2. Avoid inhaling the dust of this product and avoid contact with eyes and skin.
Storage method
Stored in a cool, dry place.
Synthesis method
1. Acetone and hydroxylamine hydrochloride method is obtained by the reaction of acetone and hydroxylamine hydrochloride. Slowly add hydroxylamine hydrochloride solution into acetone dropwise, and control the reaction temperature at 40-50°C. Neutralize the oximated reaction solution with 40% sodium hydroxide until it is alkaline (pH 7-8), cool and filter, add the filtered crude product to zeolite, distill under normal pressure, and cool to obtain a crystallized finished product.
2. Sodium nitrite method: Add 630g (8.7mol) industrial sodium nitrite and 5kg of crushed ice, stir the mixture thoroughly; slowly add sodium bisulfite solution cooled to 0~5℃ [This solution is prepared by dissolving 490g (4.7mol) anhydrous sodium carbonate in 1500ml of water, and adding sulfur dioxide until saturated] , while stirring. When all the solutions have been added, keep the temperature at 0~2°C, while stirring, add sulfur dioxide until the solution becomes acidic to the Congo red test paper, or the color of the solution fades. When passing sulfur dioxide, add 500g of ice. Transfer the mixture to a 12L flask, add 495g (625mL, 8.5mol) acetone, heat the solution to 70°C on a steam bath, stop heating, wrap the flask with asbestos cloth to reduce heat loss, and leave it overnight; then use 50 After neutralizing with % sodium hydroxide solution, separate the oil layer; extract the water layer with benzene 2 to 3 times, combine the oil layer and benzene solution, separate the water, fractionate the oil, and collect the 133~136°C fraction, which is the product , the output is 420~480g, and the yield is 67%~76%.
Purpose
1. This product is used in organic synthesis. As an analytical reagent. For the determination of cobalt. It is a raw material for medicines, pesticides, dyes and silicone coupling agents. It can also be used in analytical reagents to identify nickel, cobalt, etc.
2.Acetone oxime has strong reducing properties and can easily react with oxygen in the feed water, reducing the dissolved oxygen content in the feed water and preventing Formation of iron scale and copper scale. It can be used as boiler feed water deoxidizer in medium and high pressure boilers. When in use, control the excess acetone oxime in the water supply to 15 to 40 μg/L.
3. Used to synthesize the herbicide dibenzo-1,3-dioxa-cyclooctane-2-carboxylic acid acetone oxime ester.
