Acetyl iodide
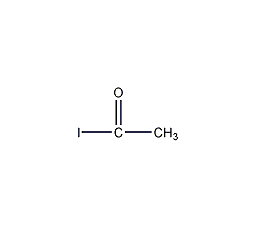

Structural formula
| Business number | 059S |
|---|---|
| Molecular formula | C2H3IO |
| Molecular weight | 169.95 |
| label |
Acetyl iodide, Iodoacetyl |
Numbering system
CAS number:507-02-8
MDL number:MFCD00000114
EINECS number:208-061-7
RTECS number:None
BRN number:1697546
PubChem ID:None
Physical property data
1. Properties: colorless and transparent liquid. There is a suffocating smell. Smokes and turns brown in the air.
2. Density (g/mL, 25/4℃): 2.0674
3. Relative density (20℃, 4℃): 2.0674
4 . Gas phase standard claims heat (enthalpy) (kJ·mol-1): -126.4
5. Boiling point (ºC, normal pressure): 109
6. Refractive index at room temperature (n20): 1.5491
7. Liquid phase standard claims heat (enthalpy) (kJ·mol-1): -163.5
8. Specific rotation (º): Undetermined
9. Autoignition point or ignition temperature (ºC): Undetermined
10. Vapor pressure (kPa, 25ºC): Undetermined
11. Saturated vapor pressure (kPa, 60ºC): Undetermined
12. Heat of combustion (KJ/mol): Undetermined
13. Critical temperature (ºC): Undetermined
14. Critical pressure (KPa): Undetermined
15. Oil and water (octanol/water) distribution Log value of coefficient: Undetermined
16. Explosion upper limit (%, V/V): Undetermined
17. Explosion lower limit (%, V/V): Undetermined
18. Solubility: Decomposes when exposed to water and ethanol.
Toxicological data
None yet
Ecological data
This substance may be harmful to the environment, and special attention should be paid to water bodies.
Molecular structure data
1. Molar refractive index: 24.31
2. Molar volume (cm3/mol): 77.4
3. Isotonic specific volume (90.2K ): 190.7
4. Surface tension (dyne/cm): 36.7
5. Polarizability (10-24cm3): 9.63
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 0.8
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: none
6. Topological molecule polar surface area 17.1
7. Number of heavy atoms: 4
8. Surface charge: 0
9. Complexity: 33
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters.��:0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Use and store according to specifications. It will not decompose and avoid contact with oxides.
2. It is corrosive and can cause burns. Avoid contact with skin when using.
Storage method
Save in a sealed, dry place and away from light.
Synthesis method
None yet
Purpose
Organic synthesis.
