Acetylacetone Acetylacetone
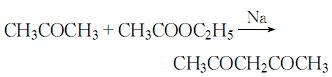
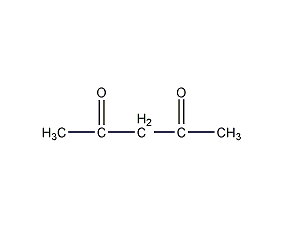
Structural formula
| Business number | 03GT |
|---|---|
| Molecular formula | C5H8O2 |
| Molecular weight | 100.12 |
| label |
diacetylmethane, 2,4-pentanedione, Diacetyl methane, 2,4-Pentanedione, 2,4-Diketopentane, ACAC |
Numbering system
CAS number:123-54-6
MDL number:MFCD00008787
EINECS number:204-634-0
RTECS number:SA1925000
BRN number:741937
PubChem number:24845859
Physical property data
1. Properties: colorless or slightly yellow transparent liquid with an ester odor.
2. Boiling point (ºC, 101.3kPa): 140.4
3. Melting point (ºC): -23.5
4. Relative density (g/mL, 25/4ºC): 0.9721
5. Relative density (g/mL, 20/20ºC): 0.9753
6. Refractive index (17ºC): 1.4541
7. Refractive index (n20ºC): 1.4494
8. Flash point (ºC): 40.56
9. Solubility: Slightly soluble in water, soluble in ethanol, ether, and chloroform Miscible with organic solvents such as acetone and glacial acetic acid.
10. Relative density (25℃, 4℃): 0.934464
11. Relative density (20℃, 4℃): 0.9702125
12. Solubility parameter (J·cm-3)0.5: 21.264
13 . van der Waals area (cm2·mol-1): 8.790×109
14. van der Waals volume (cm3·mol-1): 60.970
15. Gas phase standard combustion heat (enthalpy) (kJ·mol– 1): -2730.3
16. The gas phase standard claims heat (enthalpy) (kJ·mol-1): -380.6
17 . Liquid phase standard combustion heat (enthalpy) (kJ·mol-1): -2687.1
18. Liquid phase standard claimed heat (enthalpy) (kJ·mol -1): -423.6
19. Liquid phase standard hot melt (J·mol-1·K-1): 204.4
Toxicological data
Moderately toxic, can irritate skin and mucous membranes. When the human body is exposed to 150~300mg/kg for a long time, symptoms such as headache, nausea, vomiting, dizziness and insensibility will occur.
Ecological data
None yet
Molecular structure data
1. Molar refractive index: 25.27
2. Molar volume (cm3/mol): 105.3
3. Isotonic specific volume (90.2K ): 241.2
4. Surface tension (dyne/cm): 27.5
5. Polarizability (10-24cm3): 10.01
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 2
5. Number of tautomers: 5
6. Topological molecule polar surface area 34.1
7. Number of heavy atoms: 7
8. Surface charge: 0
9. Complexity: 82.3
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Properties: Acetylacetone is a colorless or slightly yellow flammable liquid. Boiling point 135-137℃, flash point 34℃, melting point -23℃. Relative density 0.976, refractive index n20D1.4512. 1g acetylacetone is soluble in 8g water, and is miscible with ethanol, benzene, chloroform, ether, acetone and glacial acetic acid , it decomposes into acetone and acetic acid in alkali solution. It is easy to cause combustion when exposed to high heat, open flames and strong oxidants. It is unstable in water and is easily hydrolyzed into acetic acid and acetone.
2.Moderately toxic. It can irritate the skin and mucous membranes. When the human body is exposed to (150~300)*10-6 for a long time, it will be harmed and symptoms such as headache, nausea, vomiting, dizziness and insensibility will appear. However, at a concentration of 75*10-6, it will No danger. Vacuum-tight devices should be used for production. Ventilation should be strengthened at the operation site to minimize runaway, dripping, and leaking. If poisoning occurs, leave the scene as soon as possible and breathe fresh air. Operators should wear protective equipment and undergo regular occupational disease examinations.
Storage method
1. Keep away from open flames and strong oxidants, and store in a sealed and cool place.
2. Packed in iron drums lined with plastic bags or plastic drums; 250kg per drum. Fireproof, moisture-proof, stored in dangerous goods warehouse.
Storage and transportation according to hazardous chemicals regulations.
Synthesis method
Refining method: Dissolve 20% crude acetylacetone in 80% benzene, and then shake it with an equal volume of distilled water for 3 hours. Acetic acid that is easily soluble in water is distributed into the water phase, while acetylacetone is dissolved in benzene. . Benzene is distilled off and purified to obtain acetylacetone.
1. The reaction formula of the condensation method of acetone and acetic anhydride is as follows:![]()
This route The process is simple, mature and has high yield. However, boron trifluoride is a dangerous gas, inconvenient to use, and the waste liquid treatment is difficult.
2. The reaction formula of the ethyl acetateacetone method is as follows:![]()
This method It is a classic synthesis method. This process requires metallic sodium, which brings certain risks to industrial production.
3. The reaction formula of ethyl acetoacetateacetic anhydride method is as follows:![]()
The process is simple and convenient to operate and requires less equipment investment.
However, its technical and economic indicators are not ideal and the cost of raw materials is equivalent
to the price of imported products.
4. The reaction formula of the ethyl acetoacetate-ketene method is as follows:
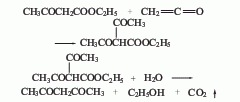
This method has mild reaction conditions and a high yield. However, the process flow is long, the price of raw materials is high, and the investment is large.
5. The reaction formula of keteneacetone condensation conversion method is as follows: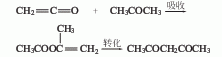
6 . The reaction formula of acetoneethyl acetate method is as follows:
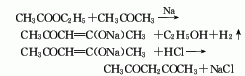
7.Pour excess refined acetic acid into Mix ethyl ester and dry acetone, cool to below 0°C, slowly add powdered sodium amide while stirring, the reaction begins, and a large amount of ammonia gas is released:
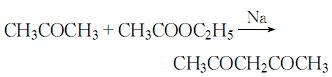
Cool with ice water and stir the reaction After 24H, let it stand at room temperature overnight. Then add equal amounts of ice and water, let it stand for separation, take the water layer, and neutralize it with dilute sulfuric acid until it becomes acidic. Then add an appropriate amount of saturated copper acetate solution to precipitate acetylacetone to form a chelate. After sufficient precipitation, filter with suction, wash with water twice, transfer to diethyl ether and shake thoroughly, add 2mol/L sulfuric acid to decompose the chelate. After standing, separate the ether layer, and the acid layer
Extract once with ether, combine the ether layers, add anhydrous calcium chloride to dry and dehydrate, filter, distill, and recover the distillable ether, collect the 125-140°C fraction, and After secondary distillation, collect the 135-140°C fraction, which is the finished product.
Purpose
1. Solvent for cellulose acetate, and its metal complex is also used as a solvent. Additives for gasoline and lubricants, drying agents for paints and varnishes. Organic synthesis intermediates.
2.Used as analytical reagents, such as extraction agents and solvents. It is also used to prepare gasoline additives, lubricants, fungicides, insecticides, and dyes.
3. Solvent. A reagent for the colorimetric determination of iron and fluorine. Determination of thallium in the presence of carbon disulfide. Determination of chromium, cobalt, hafnium, manganese and zirconium. Preparation of acetylacetonate. Extraction agent for aluminum in tungsten and molybdenum.
mily:Arial;”>Used as analytical reagents, such as extraction agents and solvents. Also used in the preparation of gasoline additives, lubricants, fungicides, insecticides, and dyes.
3 .Solvent. Reagent for colorimetric determination of iron and fluorine. Determination of thallium in the presence of carbon disulfide. Determination of chromium, cobalt, hafnium, manganese, zirconium. Preparation of acetylacetonate. Extraction agent for aluminum in tungsten and molybdenum.
