alpha-naphthoflavone
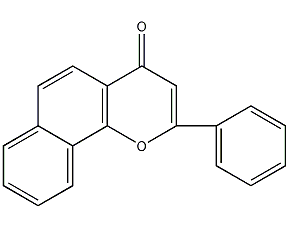

Structural formula
| Business number | 068C |
|---|---|
| Molecular formula | C19H12O2 |
| Molecular weight | 272.30 |
| label |
7,8-benzoflavonoids, benzoxanthin, 7,8-Benzoflavone |
Numbering system
CAS number:604-59-1
MDL number:MFCD00004985
EINECS number:210-071-1
RTECS number:QL6250000
BRN number:210862
PubChem number:24897748
Physical property data
Physical property data:
1. Character: Yellow flake or needle crystal
2. Melting point (℃):167℃
3. Solubility:Soluble in alcohol and concentrated sulfuric acid, the solution shows slight green fluorescence.
Toxicological data
None
Ecological data
3. Ecological data:
1. Other harmful effects: This substance may be harmful to the environment and should be harmful to water bodies. Give special attention.
Molecular structure data
5. Molecular property data: 1, Molar refractive index:82.04 2, Molar volume (m3/mol):213.3 3, Isotonic specific volume (90.2K):580.6 4, Surface tension (dyne/cm):54.8 5, Polarizability (10-24cm3 ):32.52
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 1
5. Number of tautomers: none
6. Topological molecule polar surface area 26.3
7. Number of heavy atoms: 21
8. Surface charge: 0
9. Complexity: 433
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
Properties and stability:
No decomposition products may occur under normal temperatures and pressures.
Storage method
1. Storage
Storage temperature2-8℃.
Synthesis method
2. Introduction to production methods
Will2gChalcones are soluble in120mlEthanol, heated to boiling, mixed with hydrochloric acid and refluxed24h. The residue is filtered off while hot, and the filtrate is cooled and crystallized to obtain flavanones. Then add bromine–Add flavanones to the carbon disulfide solution , benzoflavonoids are produced.
Purpose
3. Purpose
Used as a redox indicator.
