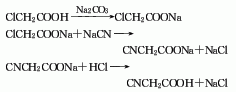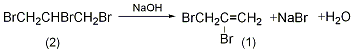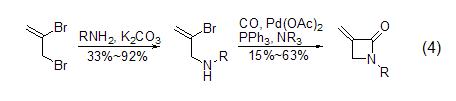
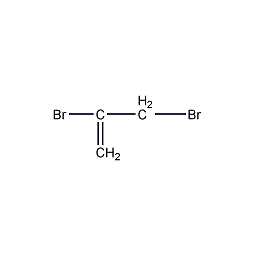
Structural formula
| Business number |
05AL |
| Molecular formula |
C3H4Br2 |
| Molecular weight |
199.87 |
| label |
2,3-dibromo-1-propene,
2,3-dibromopropene,
1,2-dibromo-2-propene
|
Numbering system
CAS number:513-31-5
MDL number:MFCD00000211
EINECS number:208-155-8
RTECS number:UC8200000
BRN number:878169
PubChem number:24861087
Physical property data
1. Properties: colorless liquid
2. Density (g/ cm3, 25/4℃): 1.93
3. Relative Density (20℃, 4℃): 2.0353
4. Melting point (ºC): Undetermined
5. Boiling point (ºC, normal pressure): 139.5
6. Boiling point (ºC, 8kPa): Undetermined
7. Refractive index: 1.544
8. Flash point (ºC): 81
9. Specific rotation (º): Undetermined
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (kPa, 25ºC): Undetermined
12. Saturated vapor pressure (kPa, 60ºC): 2.27
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC ): Undetermined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) partition coefficient: Undetermined
17. Explosion upper limit (%, V/V): Undetermined
18. Explosion lower limit (%, V/V): Undetermined
19. Solubility: Insoluble in water
Toxicological data
1. Acute toxicity: Mouse intravenous LD50: 100 mg/kg, no details except lethal dose;
2. Mutagenicity data: Microbial organism TEST system mutation: bacteria – Salmonella typhimurium Bacillus sp.: 100nmol/plate;
Ecological data
This substance may be harmful to the environment, and special attention should be paid to water bodies.
Molecular structure data
1. Molar refractive index: 30.97
2. Molar volume (cm3/mol): 99.3
3. Isotonic specific volume (90.2K ): 240.9
4. Surface tension (dyne/cm): 34.6
5. Polarizability (10-24cm3): 12.28
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 2.1
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 0
4. Number of rotatable chemical bonds: 1
5. Number of tautomers: none
6. Topological molecule polar surface area 0
7. Number of heavy atoms: 5
8. Surface charge: 0
9. Complexity: 40.2
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Use and store according to specifications. It will not decompose and avoid contact with oxides.
2. Irritating the skin, eyes and respiratory system, should be kept in a well-ventilated place.Medium operation.
3. After distillation and purification under normal pressure, there will be a small amount of decomposition, and it will become colored after standing in a glass stopper bottle.
Storage method
1. Store in a cool, dry and well-ventilated warehouse. Keep away from fire and heat sources. Protect from direct sunlight. The packaging is sealed. They should be stored separately from acids and food chemicals, and avoid mixed storage. Suitable materials should be available in the storage area to contain spills.
Synthesis method
1. Preparation method
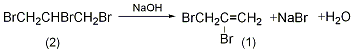
In a 500mL reaction bottle, add 200g (0.71mol) of 1,2,3-tribromopropane (2) and 10mL of water, and add solid hydroxide under stirring Sodium 50g (1.25mol). Install the distillation device and heat directly with an alcohol lamp. The alkali layer is soon partially emulsified and heated until it boils violently, and the distilled liquid is distilled out. Continue heating after the reaction has subsided. As the liquid continues to evaporate, the contents of the reaction bottle gradually become solid. Heat the brown solid until no more liquid evaporates. Wash the distillate with water and separate the oil layer. Distill under reduced pressure and collect the fractions before 95℃/10KPa, which are mainly 2,3-dibromopropene (1), which contains part of tribromopropane and water, and the remainder is tribromopropane. The crude product was dried with anhydrous calcium chloride, fractionated under reduced pressure, and the fractions at 73~76°C/10KPa were collected to obtain 105~120g of 2,3-dibromopropene (1) with a yield of 74%~84%. [1]
Purpose
1. Organic synthesis intermediates.
2. This reagent is a bifunctional reagent, and many interesting products can be prepared through different reactions of allyl and vinyl halogen atoms. 2,3-Dibromopropene can be prepared by different methods and can be further reacted to form polycyclic compounds.
Coupling reaction with organic metals The allyl and vinyl bromide atoms of 2,3-dibromopropene can be selectively substituted through continuous two-step cross-coupling. (Formula 1)[1].

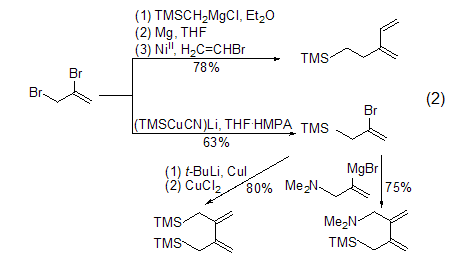
Coupling reactions are widely used in many intermediate In the synthesis of compounds, these intermediates are universal reagents for cycloaddition reactions [2~6] (Formula 2).
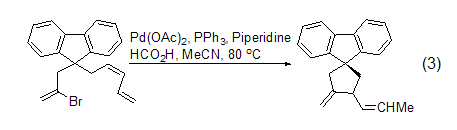
9-Fluorenyl anion and 1 -The product of the continuous alkylation reaction of bromo-2,4-pentadiene and 2,3-dibromopropene will generate a cyclization product (formula 3) under Heck reaction conditions [7].
Nucleophilic with nitrogen Reaction of reagents Under CO conditions, under the action of catalytic amounts of palladium (II) acetate and triphenylphosphine, different 2-bromopropenylamine derivatives can be used to synthesize α -Methylene-β-lactam (formula 4)[8,9].
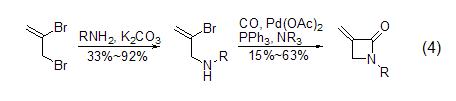
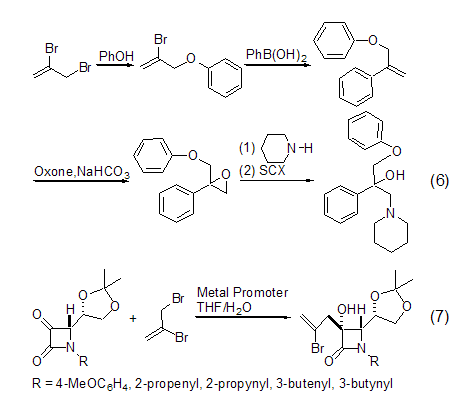
With oxygen and sulfur Reactions of Nucleophiles Intramolecular radical cyclization reactions are widely used in the organic synthesis of carbocyclic structures. For example, 2-bromoallyl ether and 2,3-dibromopropene undergo allylation reaction through phase transfer (Formula 5)[10].

In a series of small molecule alkenes In synthesis, 2,3-dibromopropene has an important application as a molecular template, and the generated olefins are further reacted to generate other products (formula 6)[11]. 2,3-Dibromopropene can also be used in stereoselective synthesis to synthesize chiral molecules, and an allylation reaction occurs simultaneously (Formula 7)[12].