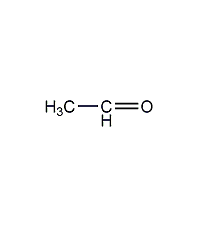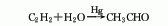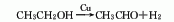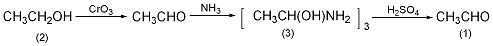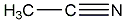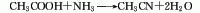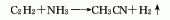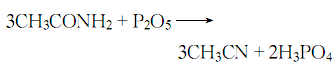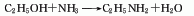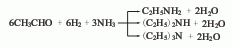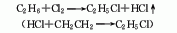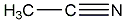
Structural formula
| Business number |
01HW |
| Molecular formula |
C2H3N |
| Molecular weight |
41.05 |
| label |
Methyl cyanide,
Cyanomethane,
Methyl cyanide,
Ethaneitrile,
synthetic raw materials
|
Numbering system
CAS number:75-05-8
MDL number:MFCD00001878
EINECS number:200-835-2
RTECS number:AL7700000
BRN number:741857
PubChem number:24856425
Physical property data
1. Properties: colorless liquid with pungent odor. [1]
2. Melting point (℃): -45[2]
3. Boiling point (℃): 81.6[3]
4. Relative density (water=1): 0.79 (15℃)[4]
5. Relative vapor density (air = 1): 1.42[5]
6. Saturated vapor pressure (kPa): 13.33 (27℃)[6]
7. Heat of combustion (kJ/mol): -1264.0[7]
8. Critical temperature (℃): 274.7[8]
9. Critical pressure (MPa): 4.83[9]
10. Octanol/water partition coefficient: -0.34[10]
11. Flash point (℃): 12.8 (CC); 6 (OC) [11]
12. Ignition temperature (℃): 524[12]
13. Explosion upper limit (%): 16.0[13]
14. Lower explosion limit (%): 3.0[14]
15. Solubility: miscible with water, soluble in most organic substances such as ethanol and ether Solvent. [15]
16. Relative density (g/mL, 20/4ºC): 0.7822
17. Relative density (g/mL, 25/ 4ºC): 0.7766
18. Relative density (g/mL, 30/4ºC): 0.77125
19. Refractive index (20ºC): 1.34411
20 .Refractive index (25ºC): 1.34163
21. Viscosity (mPa·s, 15ºC): 0.375
22. Viscosity (mPa·s, 30ºC): 0.325
23. Heat of evaporation (KJ/kg, 25ºC): 33.25
24. Heat of evaporation (KJ/kg, 80.5ºC): 29.84
25. Heat of fusion (KJ /mol): 8.17
26. Heat of formation (KJ/mol, 25ºC): 51.50
27. Heat of combustion (KJ/mol, 25ºC): 1266.09
28. Specific heat capacity (KJ/(kg·K), 25ºC, constant pressure): 1.31
29. Electrical conductivity (S/m, 25ºC): 6×10-10
30. Body expansion coefficient (K-1, 20ºC): 0.00137
Toxicological data
1. Acute toxicity[16]
LD50: 2460mg/kg (rat oral); 1250mg/kg (rabbit dermal )
LC50: 7551ppm (rat inhalation, 8h)
2. Irritation [17] Rabbit transdermal : 500mg, mild stimulation (open stimulation test)
3. Subacute and chronic toxicity [18] Cats inhale its vapor 7mg/m 3, 4 hours a day for a total of 6 months. One month after exposure to the poison, the conditioned reflex began to be destroyed. Pathological examination showed pathological changes in liver, kidney and lung.
4. Mutagenicity [19] Sex chromosome deletion and non-disjunction: Saccharomyces cerevisiae 47600ppm. Sister chromatid exchange: hamster ovary 5g/L.
5. Teratogenicity [20] Hamsters inhaled the lowest toxic dose (TCLo) 5000ppm (1h) 8 days after pregnancy, causing developmental malformations of the central nervous system. Hamsters inhaled the lowest toxic dose (TCLo) 8000ppm (1h) 8 days after pregnancy, causing developmental malformations of the musculoskeletal system.
6. Others[21] The lowest oral toxic dose in hamsters (TDLo): 300mg/kg (8 days of pregnancy), causing abnormal musculoskeletal development .
Ecological data
1. Ecotoxicity[22] LC50: 1640mg/L (96h) (fathead minnow)
2. Biological Degradability[23]
Aerobic biodegradability (h): 168~672
Anaerobic biodegradability (h) : 672~2688
3. Non-biodegradability [24]
Photooxidation half-life in water (h): 2.80 ×106~1.10×108
Photooxidation half-life in air (h): 1299~12991
Level 1 Hydrolysis half-life (h): >150000a
Molecular structure data
1. Molar refractive index: 11.22
2. Molar volume (cm3/mol): 54.9
3. Isotonic specific volume (90.2K ): 120.0
4. Surface tension (dyne/cm): 22.7
5. Polarizability (10-24cm3): 4.45
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 0
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 0
5. Number of tautomers:
6. Topological molecular polar surface area (TPSA): 23.8
7. Number of heavy atoms: 3
8. Surface charge: 0
9. Complexity: 29.3
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters Number: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
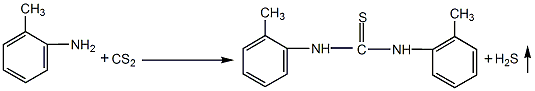
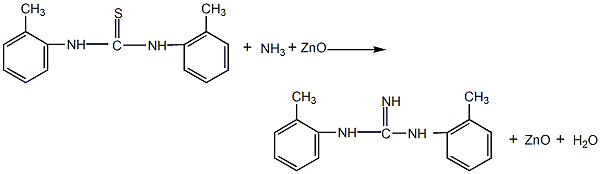
1. Chemical properties: ① Acetonitrile is a stable compound and is not easily oxidized or reduced, but there is a triple bond between carbon and nitrogen, so addition reactions are easy to occur. For example:
Addition with hydrogen halide, addition with hydrogen sulfide, addition with alcohol in the presence of inorganic acid, addition with acid or anhydride, addition with hydroxylamine.
② Hydrolysis occurs in the presence of acid or alkali to generate amide, which is further hydrolyzed to generate acid.
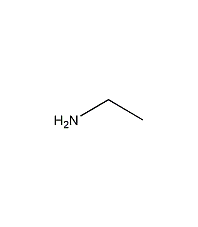
③ Reduction to generate ethylamine.
④ React with Grignard reagent, and the product is hydrolyzed to obtain ketones.
⑤ Acetonitrile can react with metallic sodium, sodium alcohol or sodium amide.
2. This product is flammable and toxic. The explosion range in air is 3.0% to 16% (volume), and the maximum allowable concentration of acetonitrile in the workplace is 70mg/m3. In acetonitrile-saturated air, rats will not die if they stay for more than 4 hours, but mice will die in 15 minutes. The oral LD50 of acetonitrile is 3.8g/kg for rats and 0.2g/kg for mice. Inhalation of acetonitrile vapor or absorption through the skin can cause poisoning, with symptoms such as nausea, vomiting, difficulty breathing, extreme fatigue and confusion, increased concentrations of cyanide and thiocyanide in the blood, and proteinuria. If acetonitrile splashes on your skin, flush it with plenty of water. If it splashes into your eyes, flush it with clean water for more than 15 minutes. If you are poisoned by inhaling acetonitrile or cyanide vapor, you should immediately move the person to fresh air and seek medical treatment. Production equipment should be sealed to prevent escape, leakage, dripping and leakage, and operators should wear protective equipment.
3. Stability[25] Stable
4. Incompatible substances[26] Acids, alkalis, strong oxidants, strong reducing agents, alkali metals, sulfuric acid, fuming sulfuric acid, chlorosulfonic acid, perchlorate
5. Polymerization hazard[27] No polymerization
6. Decomposition products[28] Hydrogen cyanide
Storage method
1. This product is packed in iron drums, with a net weight of 150kg per drum. It can also be stored and transported by truck tanker or railway tanker. The storage location should be a cool, ventilated warehouse away from fire and heat sources. The warehouse temperature should not exceed 30°C and protected from direct sunlight. Pay special attention to the integrity of the packaging to prevent poisoning caused by leakage. It should be stored separately from oxidants and acids. The lighting and ventilation facilities in the storage room should be explosion-proof, and the switch should be located outside the warehouse. Configure fire-fighting equipment of corresponding varieties and quantities. It is prohibited to use mechanical equipment and tools that are prone to sparks. Check regularly for leaks, and load and unload with care when handling to prevent damage to packaging and containers.
2. Storage precautions [29] Store in a cool, ventilated warehouse. Keep away from fire and heat sources. The storage temperature should not exceed 37°C. Keep container tightly sealed. They should be stored separately from oxidants, reducing agents, acids, alkalis, combustibles and edible chemicals, and avoid mixed storage. Use explosion-proof lighting and ventilation facilities. It is prohibited to use mechanical equipment and tools that are prone to sparks. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
There are many methods for producing acetonitrile, among which the main industrial production methods include acetic acid ammoniation method, acetylene ammoniation method and propylene ammonia oxidation by-product method.
1. Acetic acid amination method: using acetic acid and ammonia as raw materialsMaterials are reacted under the action of aluminum oxide catalyst at a temperature of 360-420°C to synthesize acetonitrile in one step. The reaction liquid is subjected to water absorption and distillation to obtain the finished product. Raw material consumption quota: acetic acid (98%) 1763kg/t, liquid ammonia (99.5%) 691kg/t.
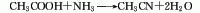
2. Acetylene amination method: Using acetylene and ammonia as raw materials and aluminum oxide catalyst, acetonitrile is synthesized in one step at a temperature of 500-600°C. Raw material consumption quota: acetylene 10231m3, liquid ammonia (99.4%) 1007kg/t.
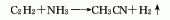
3. Propylene ammonia oxidation by-products Method: When propylene, ammonia and air are used as raw materials and acrylonitrile is synthesized through a catalyst, acetonitrile is also produced as a by-product. Each ton of acrylonitrile can produce 25-100kg acetonitrile by-product.

4. From acetamide and penta Obtained from dehydration of phosphorus oxide.
5. Obtained from the reaction of dimethyl sulfate and sodium cyanide.
Refining method: Industrial products often contain impurities such as water, hydrocarbons, acrylonitrile, acetic acid, and ammonia. During purification, the water in the crude acetonitrile is first removed by azeotropic distillation using an azeotropic mixture of acetonitrile and water, and then rectification is performed to remove high boiling point substances. For further refining, it can be dried with calcium chloride, filtered and then refluxed with 0.5% to 1% phosphorus pentoxide, and then distilled under normal pressure. Repeat this operation until phosphorus pentoxide is no longer colored (during reflux and distillation, a drying tube containing phosphorus pentoxide should be connected to prevent moisture in the air from entering), and then add newly melted potassium carbonate for distillation to remove trace amounts. of phosphorus pentoxide, and finally fractionated to obtain the pure product.
In addition to azeotropic distillation and calcium chloride dehydration, anhydrous sodium sulfate, potassium carbonate, type 4A molecular sieve or silica gel can also be used for dehydration. If there is a trace amount of unsaturated nitrile, it can be removed by refluxing with a small amount of potassium hydroxide aqueous solution (1 mL of 1% potassium hydroxide aqueous solution for every 1000 mL of acetonitrile) at the beginning. If it contains isonitrile, it can be removed by treatment with concentrated hydrochloric acid, then dried with potassium carbonate and then distilled.
6. Place absolutely dry acetamide and phosphoric anhydride in a copper container, install a fractionating column and a water condenser, heat, control the temperature so that the reaction is not too violent, and react until no distillation occurs. So far, the crude product has been collected:
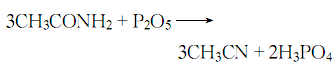
Add saturated potassium carbonate solution to the above crude product until no bubbles appear, then add a small amount of dry potassium carbonate, stir evenly, and let it stand. Pour out the crude nitrile in the upper layer and carry out fractionation together with phosphoric anhydride. The distillate at 80-82°C is collected as the finished product.
Purpose
1. Acetonitrile is the raw material for the preparation of orthoacetate, and is used to produce methyl dipermethrin and 2-chloro-3,3,3-trifluoro-1-propenyl-2,2-dimethylcyclo It is an intermediate of propane carboxylate (propane carboxylic acid ester) and can be used as a raw material for sulfonylurea herbicide intermediates and pyrimidine derivatives. It can be used in the pharmaceutical industry to produce vitamin B1 and in the synthetic rubber industry to extract the C4 fraction. agent. Used as chromatographic analysis standard material, solvent and gas chromatography stationary solution.
2. Mainly used as solvent. For example, it can be used as a solvent for extracting butadiene, a solvent for synthetic fibers and a solvent for some special coatings. Solvent used in the petroleum industry to remove tar, phenol and other substances from petroleum hydrocarbons. In the oil industry, it is used as a solvent for extracting fatty acids from animal and vegetable oils, and in medicine, it is used as a reaction medium for the recrystallization of steroid drugs. When a polar solvent with a high dielectric constant is required, a binary azeotropic mixture of acetonitrile and water is often used: containing 84% acetonitrile, boiling point 76°C. Acetonitrile is an intermediate for medicine (vitamin B1) and spices, and a raw material for manufacturing s-triazine nitrogen fertilizer synergist. Also used as a denaturant for alcohol. Used as fatty acid extraction agent, alcohol denaturant, butadiene extraction agent and solvent for acrylonitrile synthetic fiber. In addition, it can also be used to synthesize ethylamine, acetic acid, etc., and also has many uses in fabric dyeing and lighting industries.
3. Used in the preparation of vitamin B1 and other drugs and spices, and also used as fatty acid extraction agent. [30]
extended-reading:https://www.bdmaee.net/cas-1067-33-0-2/extended-reading:https://www.bdmaee.net/butylenestannonic-acid/extended-reading:https://www.newtopchem.com/archives/44383extended-reading:https://www.bdmaee.net/u-cat-sa-831-catalyst-cas111-34-2-sanyo-japan/extended-reading:https://www.cyclohexylamine.net/tetramethyl-13-diaminopropane-tmeda/extended-reading:https://www.bdmaee.net/cas-26636-01-1/extended-reading:https://www.bdmaee.net/niax-a-99/extended-reading:https://www.bdmaee.net/dabco-nem-catalyst-cas100-74-3-evonik-germany/extended-reading:https://www.bdmaee.net/author/12dma/extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/Pentamethyldiethylenetriamine-CAS3030-47-5-Jeffcat-PMDETA.pdf