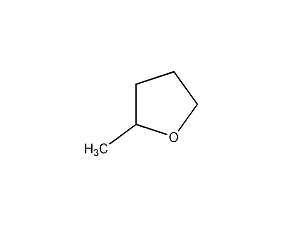
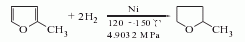γ-butyrolactone
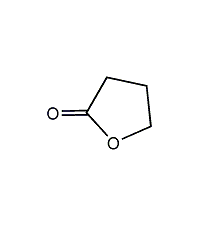
Structural formula
| Business number | 02B7 |
|---|---|
| Molecular formula | C4H6O2 |
| Molecular weight | 86 |
| label |
4-butyrolactone, 4-Hydroxybutyrolactone, 1,4-butyrolactone, 1,4-Butyrolactone, 4-Hydroxybutyric acid lactone, 4-Hydroxybutanoic acid lactone, 4-Butanolide, Ester cyclic compounds and their derivatives |
Numbering system
CAS number:96-48-0
MDL number:MFCD00005386
EINECS number:202-509-5
RTECS number:LU3500000
BRN number:105248
PubChem number:24901615
Physical property data
1. Properties: Colorless to yellow oily liquid with a cream-like aroma.
2. Relative density (15℃): 1.1286
3. Relative vapor density (g/mL, air=1): 3.0
4. Melting point (ºC): -44
5. Boiling point (ºC, normal pressure): 206
6. Viscosity (mPa·s, 25ºC): 1.7
7. Refractive index (26.5ºC): 1.4343
8. Flash point (ºC): 99.2
9. Autoignition point or ignition temperature (ºC): 455
10. Vapor pressure (mmHg, 20ºC): 1.5
11. Saturated vapor pressure (kPa, 20ºC): 2.0
12. Heat of evaporation (KJ/mol, 204ºC ): 52.3
13. Critical temperature (ºC): 457.85
14. Critical pressure (KPa): 5.131
15. Specific heat capacity (KJ/(kg ·K), 25ºC, constant pressure): 1.67
16. Specific heat capacity (KJ/(kg·K), 60ºC, constant pressure): 1.88
17. Explosion upper limit (% , V/V): 16
18. Lower explosion limit (%, V/V): 1.4
19. Solubility: miscible with water, also soluble in methanol and ethanol , ether, benzene and other organic solvents.
20. Relative density (20℃, 4℃): 1.1299
21. Refractive index at room temperature (n20): 1.4346
22. Refractive index at room temperature (n25): 1.4348
23. Solubility parameter (J·cm-3)0.5 : 25.593
24. van der Waals area (cm2·mol-1): 6.250×109
25. van der Waals Volume (cm3·mol-1): 45.890
26. Liquid Phase standard hot melt (J·mol-1·K-1): 140.0
Toxicological data
1. Acute toxicity: oral administration – rat LD50: 1540 mg/kg; oral administration – mouse LD50: 1720 mg/kg
2. It is of low toxicity. Irritating to skin�, easily absorbed by the skin, contact with skin should be avoided.
Ecological data
This substance is slightly hazardous to water.
Molecular structure data
1. Molar refractive index: 20.18
2. Molar volume (cm3/mol): 76.2
3. Isotonic specific volume (90.2K ): 186.0
4. Surface tension (dyne/cm): 35.4
5. Dielectric constant:
6. Dipole moment (10-24cm3):
7. Polarizability: 8.00
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: 2
6. Topological molecule polar surface area 26.3
7. Number of heavy atoms: 6
8. Surface charge: 0
9. Complexity: 67.9
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Avoid contact with strong oxidants, strong acids, strong bases, and strong reducing agents.
Soluble in water, methanol, etc., non-corrosive to metals, flammable, low-toxic, easily absorbed by the skin, avoid contact with skin.
Chemical properties: It is a relatively stable compound. It is prone to hydrolysis under the action of hot alkali. The hydrolysis is reversible. When pH=7, lactone is generated again. Hydrolysis is slow in acidic media.
2.This product is a low-toxicity substance that has an anesthetic effect on the central nervous system. The oral LD50 of rats is 1800mg/kg, and it is harmful to the skin. Irritating effect, its smoke is irritating to the eyes, mucous membranes and upper respiratory tract.
3. Exist in flue-cured tobacco leaves, burley tobacco leaves, oriental tobacco leaves, and smoke.
4. Found in apricots, bread, coffee, and fried Volatile aroma components of hazelnuts.
5. Preparation method:
Add dry 2 mL of acetonitrile, 110 mg (1.0 mmol) of trimethylsilyl chloride, 187 g (1.1 mmol) of silver nitrate were added at 0°C under nitrogen protection, and the reaction was stirred for 1 hour. Pour out the supernatant (remove silver chloride), add 150 mg CrO3 (1.5 mmol) to 1 mL of acetonitrile with stirring, and stir for 15 minutes to react to obtain TMSONO2·CrO3 oxidant. A solution of THF (2) (1.0 mmol) dissolved in 1 mL acetonitrile was slowly added dropwise while cooling in an ice water bath, and the reaction was stirred at room temperature for 24 hours. Filter, wash with dichloromethane, and evaporate the solvent to obtain crude product ① (1). Silica gel column purification, yield 65%. Note: ① The possible reaction mechanism of this reaction is as follows. (Reference book page 494)[1]
Storage method
1. Store in a cool, ventilated warehouse. Keep away from fire and heat sources. They should be stored separately from oxidants, acids, alkalis, and food chemicals, and avoid mixed storage. Equipped with the appropriate variety and quantity of fire equipment. The storage area should be equipped with emergency spill treatment equipment and suitable containment materials.
2. It can be stored and transported in ordinary steel containers. It cannot be stored and transported in rubber, phenolic resin, epoxy resin, concrete and sharp equipment. The color will turn slightly yellow after long-term storage in steel containers. During storage and transportation, keep away from fire and heat sources, prevent direct sunlight, and keep containers sealed. Store in a dry, cool, ventilated space and separate from oxidants, acids and alkalis.
Synthesis method
1. Maleic anhydride hydrogenation method This method is an advanced process developed in the 1970s. It uses a one-stage hydrogenation reaction to produce tetrahydrofuran and γ-butyrolactone in any ratio. The usual ratio is tetrachlorofuran: γ-butyrolactone. Lactone=3-4:1. There are many manufacturing enterprises, but the scale is small, with an average capacity of 300t/a. The production capacity of this method accounts for 30% of the total domestic production capacity.

2, 1,4-D The glycol dehydrogenation method uses a tubular reactor filled with a flake copper catalyst (with zinc oxide as a carrier). The reaction temperature is controlled at 230-240°C. The crude γ-butyrolactone, the reaction product, is distilled under reduced pressure to obtain the finished product, with a yield of more than 77%.
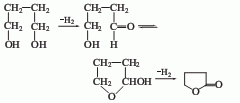
3. So 1, 4 -Butanediol is used as raw material, preheated first, reacted with hydrogen in the presence of copper catalyst, and the temperature is controlled at 230-240°C to obtain crude γ-butyrolactone, which is obtained by distillation under reduced pressure.
4. At present, succinic anhydride has been obtained by hydrogenation using the maleic anhydride method, and then further hydrogenated and dehydrated to obtain the product.
5. Allyl alcohol method: Use aromatic hydrocarbons as solvents and rhodium complexes as catalysts to hydroformylate allyl alcohol and raw gas (H2+CO), and use skeleton nickel to catalyze hydrogenation to obtain 1,4-butanediene. alcohol, and then produce γ-butyrolactone, and co-produce tetrahydrofuran.
6. Furfural method: DuPont decarbonylates furfural in water vapor to obtain furan, which is further oxidized to generate γ-butyrolactone.
7. Ethylene acetic acid method B.and acetic acid at 120°C, catalyzed by MPa and manganese acetate or manganese dioxide, to produce finished products.
8. β-Formylpropionate method β-Formylpropionate is obtained by hydrogenation reduction, hydrolysis, dehydration and cyclization.
Refining method: dry anhydrous calcium sulfate and then fractionate. It can also be refined by steam distillation.
9. Tobacco: FC, 9, 18, 40; BU, OR, 40; intramolecular esterification of γ-hydroxybutyric acid; produced from vinyl acetic acid; produced from 1,4-butanediol Prepared by dehydrogenation; produced from maleic anhydride, with tetrahydrofuran as a by-product.
10. Preparation method:
In a reaction flask equipped with a stirrer, reflux condenser (connected to a gas pipe to the hydrogen chloride absorption device), thermometer, and dropping funnel, add chloroform 250mL, 250g (1.88mol) anhydrous aluminum trichloride, stir to form a suspension. Slowly add a mixture of 42.5g (0.528mol) chloroethanol and 80g (0,5mol) diethyl malonate (2) dropwise, and complete the addition in about 2 hours. Hydrogen chloride gas is continuously produced during the reaction. Leave overnight. Under cooling in an ice-water bath, slowly add 250 mL of 2 mol/L hydrochloric acid dropwise to decompose the reaction solution, and generate hydrogen chloride gas at the same time. After the addition is completed, the temperature is slowly raised and the reaction is refluxed for 40 hours. Separate the brown-red chloroform liquid (retain the aqueous layer), and dissolve the solid in 150 mL of water. The aqueous layers were combined and extracted three times with chloroform. Combine the chloroform layers, wash with water until neutral, and dry over anhydrous calcium chloride. Chloroform was evaporated, and then distilled under reduced pressure. The fractions at 85-100°C/1.05kPa were collected to obtain 36g of γ-butyrolactone (1), with a yield of 82%. [1]
Purpose
Used for gas chromatography stationary solution (maximum operating temperature 30°C, solvent is methanol). Separate and analyze hydrocarbons, various oxygen-containing compounds, and permanent gases. Synthesize intermediates such as polyvinylpyrrolidone, DL-methionine, hexahydropyridine, phenylbutyric acid and thioacetic acid. Solvent for polyacrylonitrile, acetate, polymethylmethacrylate and polystyrene. It is also an ideal antioxidant, plasticizer, extractant, absorbent, dispersant, fixative, and coagulant; it can be used as an anesthetic and sedative in the pharmaceutical industry, and can be used to synthesize ciprofloxacin and interferon, etc. It is an intermediate for vitamins, cyclopropylamine, etc.; it is also widely used in agriculture and forestry, and is an intermediate for the production of plant growth agents, pesticides, etc. In addition, it can also be used for making batteries, capacitors, color rolls, etc. γ-Butyrolactone is a high-boiling point solvent with strong solubility, non-toxicity, safe and convenient use and management. It is used as an extraction agent for butadiene, aromatic hydrocarbons and high-grade greases in petroleum processing; it is also used in the chemical fiber industry. It is a spinning solvent for acrylic fiber and a dyeing auxiliary for wool, nylon, acrylic and other fibers. In organic synthesis, GBL is also widely used. It is a raw material for the synthesis of α-pyrrolidone, N-methylpyrrolidone, vinylpyrrolidone, acetyl-γ-butyrolactone, cyclopropylamine, etc. It is also a raw material for the synthesis of pesticides and herbicides. Intermediates of agents, drugs Naofukang, ciprofloxacin, vitamin B1, chlorophyll, etc. It is also used in the synthesis of chlorophenoxybutyric acid herbicides and plant growth regulators. Used as a plasticizer for epoxy resin adhesives and also as a solvent. It is widely used as a solvent for resins, high molecular polymers, petroleum products, coatings, inks, dyes and agricultural chemicals. It is also used as lubricating oil additive, dyeing auxiliary, fuel oil viscosity regulator, acrylic fiber coagulant, photographic agent dispersant, thickener, aromatic hydrocarbon extractant and conductive solvent. In organic synthesis, it is used to manufacture 2-pyrrolidone, N-methyl-2-pyrrolidone, polyvinylpyrrolidone and butyric acid, etc. In the food industry, it can be used as a clarifying agent for liquor and beer and for preparing fruit and meat flavors.
extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/31-12.jpgextended-reading:https://www.newtopchem.com/archives/category/products/page/141extended-reading:https://www.cyclohexylamine.net/amine-catalyst-b16-soft-foam-amine-catalyst-b16/extended-reading:https://www.cyclohexylamine.net/low-odor-catalyst-pt302-dabco-hard-foam-catalyst/extended-reading:https://www.newtopchem.com/archives/582extended-reading:https://www.bdmaee.net/nt-cat-ncm-catalyst-cas110-18-9-newtopchem/extended-reading:https://www.newtopchem.com/archives/40462extended-reading:https://www.bdmaee.net/niax-nmm-tertiary-amine-catalysts-momentive/extended-reading:https://www.morpholine.org/tetrachloroethylene-perchloroethylene-cas127-18-4/extended-reading:https://www.bdmaee.net/wp-content/uploads/2016/05/Lupragen-N205-MSDS.pdf