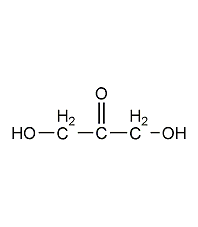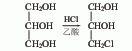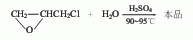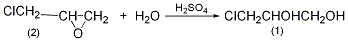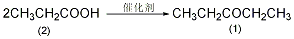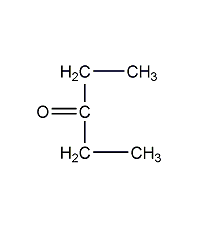
Structural formula
| Business number |
02AR |
| Molecular formula |
C5H10O |
| Molecular weight |
86 |
| label |
dimethylacetone,
diethyl ketone,
Diethyl ketone,
Dimethylacetone,
Propione,
aliphatic compounds
|
Numbering system
CAS number:96-22-0
MDL number:MFCD00009320
EINECS number:202-490-3
RTECS number:SA8050000
BRN number:635749
PubChem number:24856370
Physical property data
1. Properties: colorless liquid with acetone odor. [1]
2. Melting point (℃): -42~-39[2]
3. Boiling point ( ℃): 102[3]
4. Relative density (water=1): 0.81[4]
5 .Relative vapor density (air=1): 3.0[5]
6. Saturated vapor pressure (kPa): 3.49 (20℃)[6] sup>
7. Heat of combustion (kJ/mol): -3100.2[7]
8. Critical temperature (℃): 288.3 [8]
9. Critical pressure (MPa): 3.729[9]
10. Octanol/water partition coefficient: 0.99 [10]
11. Flash point (℃): 13 (OC) [11]
12. Ignition Temperature (℃): 452[12]
13. Explosion limit (%): 3[13]
14 .Lower explosion limit (%): 1.6[14]
15. Solubility: slightly soluble in water, miscible in ethanol and ether, soluble in acetone. [15]
16. Heat of evaporation (KJ/mol): 33.75
17. Heat of fusion (KJ/mol): 11.60
18. Heat of formation (KJ/mol): 258.8
19. Specific heat capacity (KJ/(kg·K), 25ºC, constant pressure): 2.22
20. Heat Conductivity (W/(m·K), 20ºC): 0.14235
21. Volume expansion coefficient (K-1): 0.00113
22. Critical density (g·cm-3): 0.26
23. Critical volume (cm3·mol-1 ): 331
24. Critical compression factor: 0.264
25. Eccentricity factor: 0.350
26. Solubility parameter (J·cm– 3)0.5: 18.260
27. van der Waals area (cm2·mol-1 ): 8.540×109
28. van der Waals volume (cm3·mol-1): 59.500
29. Gas phase standard combustion heat (enthalpy) (kJ·mol-1): -3138.75
30. Gas phase standard claimed heat (enthalpy) ( kJ·mol-1): -257.95
31. Gas phase standard entropy (J·mol-1·K-1): 370.10
32. Gas phase standard formation free energy (kJ·mol-1): -134.3
33. Gas phase standard hot melt ( J·mol-1·K-1): 129.87
34. Liquid phase standard combustion heat (enthalpy) (kJ·mol -1): -3100.19
35. Liquid phase standard claims heat (enthalpy) (kJ·mol-1): -296.51
36. Liquid phase standard entropy (J·mol-1·K-1): 266.01
37. Liquid phase standard free energy of formation ( kJ·mol-1): -142.09
38. Liquid phase standard hot melt (J·mol-1·K-1 ):190.9
Toxicological data
1. Skin/eye irritation
Open irritation test: rabbit, skin contact: 410mg, severity of reaction: mild.
Standard Draize test: rabbit, skin contact: 500mg/24H, severity of reaction: mild.
Standard Draize test: Rabbit, eye contact: 50 mg, severity of reaction: moderate.
Standard Draize test: Rabbit, eye contact: 100mg/24H, severity of reaction: moderate.
2. Acute toxicity: rat oral LD50: 2140mg/kg; rat inhalation LCLo: 8000ppm/4H; rat abdominal LDLo: 1250mg/kg; mouse oral LD50: 3100mg/ kg; mouse intravenous LD50: 513mg/kg; rabbit skin contact LD50: 20mL/kg;
3. Mutagenicity
Yeast gene conversion and mitotic recombination experiments: 14800ppm ;
Yeast sex chromosome loss and non-disjunction experiment: 14800ppm;
4. The olfactory threshold concentration is 33mg/m3. The maximum allowable concentration in the workplace is 705mg/m3 (USA).
5. Acute toxicity [16] LD50: 2140mg/kg (rat oral); 20000mg/kg (rabbit transdermal)
p>
6. Irritation [17]
Rabbit transdermal: 500mg (24h), mild irritation.
Rabbit eye: 50mg, moderate irritation.
7. Subacute and chronic toxicity [18] Rats ingested 250mg or 454mg/(kg·d) through drinking water, a total of 13 Months later, no other abnormal reactions were seen except for a slight weight loss.
Ecological data
1. Ecotoxicity No data available
2. Biodegradability No data available
3 .Non-biodegradability[19] In the air, when the concentration of hydroxyl radicals is 5.00×105/cm3 When, the degradation half-life is 8d (theoretical).
Molecular structure data
1. Molar refractive index: 25.24
2. Molar volume (cm3/mol): 108.1
3. Isotonic specific volume (90.2K ): 236.1
4. Surface tension (dyne/cm): 22.6
5. Dielectric constant:
6. Dipole moment (10-24cm3):
7. Polarizability: 10.00
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 0.9
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 2
5. Number of tautomers: 2
6. Topological molecule polar surface area 17.1
7. Number of heavy atoms: 6
8. Surface charge: 0
9. Complexity: 41.9
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Stability[20] Stable
2. Incompatible substances [21] Strong oxidants, strong reducing agents, strong bases
3. Polymerization hazards[22] No aggregation
Storage method
Storage Precautions[23] Store in a cool, ventilated warehouse. Keep away from fire and heat sources. The storage temperature should not exceed 37℃. Keep container tightly sealed. They should be stored separately from oxidants, reducing agents and alkalis, and avoid mixed storage. Use explosion-proof lighting and ventilation facilities. It is prohibited to use mechanical equipment and tools that are prone to sparks. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
1. Obtained from the oxidation of 3-pentanol. The oxidation reaction is carried out at 90°C, filtered after the reaction, and then fractionated to collect the 101-104°C fraction, which is the finished product.

2. Its preparation method is: It is obtained by oxidation of 3-pentanol. It is commonly used to oxidize sodium dichromate in the presence of sulfuric acid. The oxidation temperature is 90°C. After the reaction is completed, the finished product is obtained by distillation.
Refining method: dry with barium oxide or calcium sulfate and then distill. You can also use calcium chloride to reflux for 2 hours, add fresh calcium chloride and leave it overnight, filter and then distill. High-purity 3-pentanone can be distilled in a distillation tower with a theoretical plate number of 100 at 93.33KPa and a reflux ratio of 100:1. The distillate is crystallized step by step, and calcium hydroxide is added for redistillation. The purity can be Up to 99.95%±0.01%.
3. Preparation method:
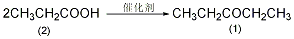
Preparation of manganese carbonate-pumice catalyst: Dissolve 70g manganese chloride tetrahydrate (0.35mol) in 100mL water, slowly add 38g anhydrous sodium carbonate (0.35mol) dissolved in 120mL water while stirring The solution. Filter the generated manganese carbonate precipitate and wash thoroughly with water. Transfer the solid matter to a large evaporating dish and add appropriate amount of water to make it viscous. Add an appropriate amount of pumice (4 to 8 mesh) and stir to make most of the sticky substance adhere to the pumice. 2-Pentanone (1): Heat the above catalyst in a heating tube at 360-400°C for 8 hours under nitrogen protection to convert manganese carbonate into manganese oxide. At 350~400℃��740g (10mL) of re-distilled propionic acid (2) was added dropwise to the catalyst from the dropping funnel at a rate of about 30 drops/min. It takes about 48 to 72 hours to complete the addition. The distilled liquid separated into two layers. The organic layer was separated, the aqueous layer was saturated with solid potassium carbonate, and the organic layer was separated. The organic layers were combined and treated with solid potassium carbonate to remove possible acid and water. Filter, fractionate, and collect the fractions at 101 to 103°C to obtain 250 g of 3-pentanone (1). The yield is 29%. [25]
Purpose
Used in medicine and organic synthesis. [24]
extended-reading:https://www.bdmaee.net/nnn-trimethyl-n-hydroxyethyl-bisaminoethyl-ether-cas-83016-70-0-jeffcat-zf-10/extended-reading:https://www.newtopchem.com/archives/40230extended-reading:https://www.bdmaee.net/fascat-4233-catalyst/extended-reading:https://www.bdmaee.net/toyocat-dt-strong-foaming-catalyst-pentamethyldiethylenetriamine-tosoh/extended-reading:https://www.bdmaee.net/niax-dmea-catalysts-dimethylethanolamine-momentive/extended-reading:https://www.cyclohexylamine.net/low-odor-tertiary-amine-catalyst-dabco-low-odor-tertiary-amine-catalyst/extended-reading:https://www.bdmaee.net/bismuth-neodecanoate-cas34364-26-6-bismuth-neodecanoate/extended-reading:https://www.newtopchem.com/archives/1891extended-reading:https://www.newtopchem.com/archives/category/products/page/145extended-reading:https://www.cyclohexylamine.net/dabco-blx-11-polyurethane-foaming-catalyst/