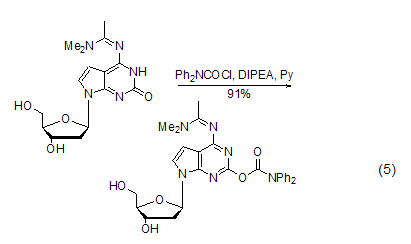
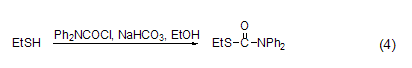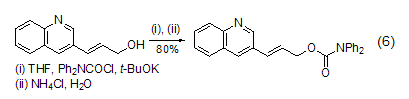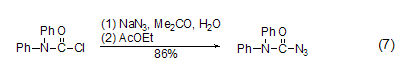Prednisone
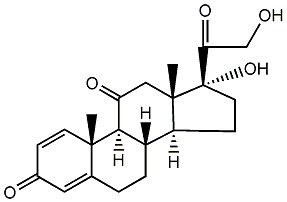
Structural formula
| Business number | 015T |
|---|---|
| Molecular formula | C21H26O5 |
| Molecular weight | 358.43 |
| label |
prednisone tablets, 17α,21-dihydroxy-1,4-pregnadiene-3,11,20-trione, Dehydroadrenocorticoid hormone, dehydrocortisone, prednisone, dehydrocortisone, 1,4-Pregnadiene-17α,21-diol-3,11,20-trione, 1-Cortisone, 17α,21-Dihydroxy-1,4-pregnadiene-3,11,20-trione, Dehydrocortisone |
Numbering system
CAS number:53-03-2
MDL number:MFCD00003608
EINECS number:200-160-3
RTECS number:TU4154100
BRN number:2065301
PubChem number:24898748
Physical property data
1. Characteristics: White or off-white crystalline powder. Odorless. Slightly persistent bitterness
2. Density ( g/mL,25/4℃): Undetermined
3. Relative steam Density (g/mL,AIR=1): Undetermined
4. Melting point ( ºC):233~235
5. Boiling point ( ºC,Normal pressure): Undetermined
6. Boiling point ( ºC, 13.33kpa):
7. Refractive index: Undetermined
8. Flashpoint (ºC): Undetermined
9. Specific optical activity Degree (º, C=1, DIOXANE): 172
10. Spontaneous ignition point or ignition temperature (ºC): Undetermined
11. Vapor Pressure (kPa,25ºC): Undetermined
12. saturated vapor pressure (kPa,60ºC): Undetermined
13. Burning heat (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical Pressure (KPa): Undetermined
16. Oil and water ( Octanol/Log value of water) partition coefficient: Undetermined
17. Explosion limit (%,V/V): Undetermined
18. Lower explosion limit (%,V/V): Undetermined
19. Solubility :1gThe product dissolves in approximately150mlEthanol, about200ml Chloroform, Slightly soluble in methanol and dioxane, very slightly soluble in water.
Toxicological data
1, acute toxicity: For women, TDLo: 2400ug/kg/2D-I; for men, TDLo: 857ug/kg; Men take oral LDLo: 400ug/kg;FemaleTDLo:113mg/kg;
Mouse abdominal cavityLD50: 135 mg/kg; Mouse subcutaneous LD50: 101 mg/kg; mouse intramuscular LD50: 600 mg/kg
2, carcinogenicity: rat abdominal cavity TDLo: 860mg/kg/26W-I
3, reproductive toxicity: female mice subcutaneous TDLo: 24mg/kg, 13 -18The Queen Conceives
4, mutagenic microorganismsTEST system: bacteria –Salmonella typhimurium: 3333ug/plate;
MutationmicroorganismsTESTSystem: Bacteria – Salmonella Typhimurium: 333ug/plate;
Ecological data
None yet
Molecular structure data
1. Molar refractive index: 94.08
2. Molar volume (m3/mol):273.6
3. isotonic ratio(90.2K):757.0
4. Surface Tension(dyne/cm):58.5
5. Polarizability(10-24cm3)���37.29
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP):1.5
2. Number of hydrogen bond donors: 2
3. Number of hydrogen bond acceptors: 5
4. Number of rotatable chemical bonds: 2
5. Number of tautomers: 27
6. Topological molecular polar surface area (TPSA):91.7
7. Number of heavy atoms: 26
8. Surface charge: 0
9. Complexity: 764
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 6
12. The number of uncertain atomic stereocenters: 0
13. Determine the number of stereocenters of chemical bonds: 0
14. Uncertain number of chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
None yet
Storage method
Mainly used for various acute severe bacterial infections, severe allergic diseases, collagen diseases (lupus erythematosus, nodular periarteritis, etc.), rheumatism, rheumatoid arthritis , nephrotic syndrome, severe bronchial asthma, thrombocytopenic purpura, granulocytopenia, acute lymphoblastic leukemia, various adrenocortical insufficiency, exfoliative dermatitis, pemphigus, neurodermatitis, eczema, etc.
Synthesis method
Made from cortisone as raw material.
Purpose
Mainly used for various acute severe bacterial infections, severe allergic diseases, collagen diseases (lupus erythematosus, nodular periarteritis, etc.), rheumatism, rheumatoid arthritis, nephrotic syndrome, severe bronchial asthma, platelet Reducing purpura, granulocytopenia, acute lymphoblastic leukemia, various adrenocortical insufficiencies, exfoliative dermatitis, pemphigus, neurodermatitis, eczema, etc.
extended-reading:https://www.newtopchem.com/archives/1006extended-reading:https://www.bdmaee.net/dibutyldichlorotin/extended-reading:https://www.newtopchem.com/archives/1093extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/Polyurethane-Catalyst-A33-CAS-280-57-9–33-LV.pdfextended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/52.jpgextended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/Organic-mercury-replacement-catalyst-NT-CAT-E-AT.pdfextended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/Dibutyl-tin-maleate-CAS78-04-6-tributyl-tin-oxide.pdfextended-reading:https://www.bdmaee.net/fascat-4210-catalyst/extended-reading:https://www.newtopchem.com/archives/category/products/page/159extended-reading:https://www.newtopchem.com/archives/43001