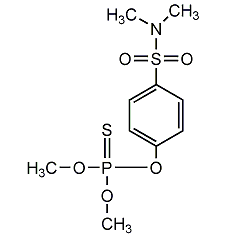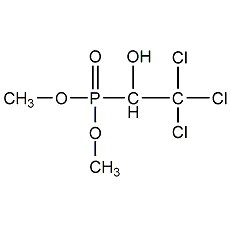
Structural formula
| Business number |
015M |
| Molecular formula |
C4H8Cl3O4P |
| Molecular weight |
257.44 |
| label |
Dimethyl-(1-hydroxy-2,2,2-trichloroethyl)phosphonate,
O,O-dimethyl-(2,2,2-trichloro-1-hydroxyethyl)phosphate,
metrefosate,
Metrifonate,
Chloroftalm,
Chlorophos,
Chlorophthalm,
Chloroxyphos,
Combot,
Dipterex,
DEP,
Dimethyl-(1-hydroxy-2,2,2-trichloroethyl)phosphonate,
Organophosphorus pesticides
|
Numbering system
CAS number:52-68-6
MDL number:MFCD00055272
EINECS number:200-149-3
RTECS number:TA0700000
BRN number:1709434
PubChem number:24899252
Physical property data
1. Characteristics: The pure product is white crystal with an aldehyde odor. [1]
2. Melting point (℃): 83~84[2]
3. Boiling point (℃) : 100 (13.3Pa) [3]
4. Relative density (water = 1): 1.73[4]
5. Saturated vapor pressure (kPa): 13.33 (100℃)[5]
6. Octanol/water partition coefficient: 0.51[6]
7. Solubility: soluble in water and chloroform, insoluble in gasoline. [7]
Toxicological data
1. Acute toxicity[8]
LD50: 400~900mg/kg (rat oral); 500mg/kg (rabbit Transdermal)
2. Irritation [9] Rabbit eye: 120 mg (6 days, intermittent), mild irritation.
3. Mutagenicity [10] Microbial mutagenicity: Salmonella typhimurium 3400nmol/dish. Mammalian somatic cell mutagenesis: mouse lymphocytes 80mg/L. Sister chromatid exchange: hamster lung 20mg/L. Unprogrammed DNA synthesis: human fibroblasts 100mg/L. Cytogenetic analysis: human leukocytes 40mg/L.
4. Teratogenicity [11] The lowest toxic dose (TDLo) of 1450 mg/kg was administered orally to rats 6 to 15 days after pregnancy, causing Developmental malformations of the central nervous system, craniofacial (including nose, tongue), and musculoskeletal systems. The lowest toxic dose (TDLo) of 3g/kg was administered orally to mice 7 to 16 days after pregnancy, causing developmental malformations of the genitourinary system.
5. Carcinogenicity [12] IARC Carcinogenicity Comment: G3, insufficient evidence of carcinogenicity to humans and animals.
6. Others[13] The lowest oral toxic dose in rats (TDLo): 1450mg/kg (gestation 6~15d), causing central nervous system Abnormalities in nervous system development, craniofacial development, and musculoskeletal development.
Ecological data
1. Ecotoxicity[14]
LC50: 1.4mg/L (96h) (rainbow trout); 0.94mg/L ( 96h) (Bluegill sunfish)
EC50: 0.00018mg/L (48h) (Water flea)
2. Biodegradability[15]
Aerobic biodegradation (h): 24~1080
Anaerobic biodegradation (h): 96~4320
3. Non-biodegradability[16]
Photolysis maximum light absorption (nm): <200
In air Photooxidation half-life (h): 1~101
First-level hydrolysis half-life (h): 68
Molecular structure data
1. Molar refractive index: 46.95
2. Molar volume (cm3/mol): 163.5
3. Isotonic specific volume (90.2K ): 427.2
4. Surface tension (dyne/cm): 46.6
5. Polarizability (10-24cm3): 18.61 p>
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 4
4. Number of rotatable chemical bonds: 3
5. Number of tautomers: none
6. Topological molecule polar surface area 55.8
7. Number of heavy atoms: 12
8. Surface charge: 0
9. Complexity: 183
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 1
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Easy to hydrolyze and dehydrochloride, heat, decompose quickly when >pH 6, and photolyze slowly. It is quickly converted into dichlorvos by alkali. When hydrolyzed at 22°C, the half-life shortens as the pH value increases.
2. Stability[17] Stable
3. Incompatible substances[18] Strong oxidizing agent, strong alkali
4. Conditions to avoid contact[19] Heating
5. Polymerization hazard[20] No polymerization
6. Decomposition products[21] Hydrogen chloride, phosphorus oxide
Storage method
Storage Precautions[22] Store in a cool, ventilated warehouse. Keep away from fire and heat sources. The packaging is sealed. They should be stored separately from oxidants and alkalis, and avoid mixed storage. Equipped with the appropriate variety and quantity of fire equipment. Suitable materials should be available in the storage area to contain spills.
Synthesis method
1. Methanol and trichloroacetaldehyde are mixed to form hemiacetal. Then, at low temperature, phosphorus trichloride reacts with methanol to obtain dimethyl phosphite. At a higher temperature, trichloroacetaldehyde reacts with dimethyl phosphite. Dimethyl phosphate undergoes a condensation reaction to form trichlorfon.
2. First, methanol and trichloroacetaldehyde are mixed to form hemiacetal (and containing a small amount of acetal). Then at a lower temperature, phosphorus trichloride reacts with methanol to generate dimethyl phosphite (free methanol or hemiacetal and methanol in acetal can easily react with phosphorus trichloride), and HCl and CH3Cl are eliminated in time. Finally, at higher temperatures, a condensation reaction occurs between trichloroacetaldehyde and dimethyl phosphite to form trichlorfon. In continuous operation, the methanol and trichloroacetaldehyde are controlled to be slightly larger than the theoretical amount. The mass ratio is phosphorus trichloride: methanol: trichloroacetaldehyde = 1: (0.73~0.78): (1.12~1.18); the esterification temperature is 40 ~50℃, residence time 10min; the liquid phase temperature of the deacidification liquid is 80℃, the condensation temperature is 90~95℃, the residence time is 40~50min; the temperature of the deacidification liquid is first 95~105℃, and then <120℃. Trichlorfon production can also be carried out by one-step synthesis and in stages. That is, feeding once, dimethyl phosphite is generated at low temperature, and trichlorfon is directly generated at high temperature without separation. In the esterification stage, trichloroacetaldehyde and methanol are mixed, the temperature does not exceed 50°C, cooled to below 5°C, phosphorus trichloride is added, and hydrogen chloride and methyl chloride are discharged for recycling. In the condensation stage, after the esterification liquid further removes hydrogen chloride through the spinner, it enters the reaction tank at a temperature of 80 to 120°C to generate trichlorfon.
Purpose
1. Used as pesticide. It is suitable for the prevention and control of chewing mouthpart pests on rice, wheat, vegetables, tea trees, fruit trees, mulberry trees, cotton and other crops, as well as livestock parasites and sanitary pests.
2. An organophosphorus pesticide. It is a highly efficient, low-toxic, low-residue, broad-spectrum insecticide. It is mainly gastrotoxic, has both contact and penetrating activity. It has a wide range of applications in agriculture and is used to control various pests such as cabbage caterpillars, cotton leaf springtails, wild mulberry silkworms, mulberry vines, weevils, fruit leaf wasps, fruit flies and other pests. Refined trichlorfon can be used to control internal and external parasites in pigs, cattle, horses, and mules, and is effective against household and environmental health pests. It can be used to treat schistosomiasis and is a good multi-effect anthelmintic in animal husbandry. Trichlorfon has contact and stomach poisoning effects and penetrating activity. The raw powder can be processed into powders, wettable powders, soluble powders and emulsions and other dosage forms. It can also be directly prepared into aqueous solutions or made into poisonous baits for the prevention and treatment of chewing mouthparts and piercing mouthparts in agriculture, forestry and horticulture. Pests, underground pests, etc.
3. Used as pesticide. [23]
extended-reading:https://www.newtopchem.com/archives/631extended-reading:https://www.newtopchem.com/archives/44131extended-reading:https://www.bdmaee.net/dibutyltin-dilaurate-cas77-58-7-dibutyl-tin-dilaurate/extended-reading:https://www.cyclohexylamine.net/category/product/page/12/extended-reading:https://www.cyclohexylamine.net/catalyst-9727-polyurethane-catalyst-9727/extended-reading:https://www.bdmaee.net/jeffcat-dmea-catalyst-cas107-15-3-huntsman/extended-reading:https://www.newtopchem.com/archives/1850extended-reading:https://www.bdmaee.net/pc-cat-dmcha-catalyst/extended-reading:https://www.bdmaee.net/toyocat-dmch-hard-bubble-catalyst-for-tertiary-amine-tosoh/extended-reading:https://www.newtopchem.com/archives/1594