Barium acetate
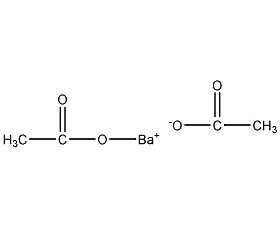

Structural formula
| Business number | 05M5 |
|---|---|
| Molecular formula | BaC4H6O2 |
| Molecular weight | 255.42 |
| label |
barium acetate, high purity compounds, catalyst, mordant, Precipitant |
Numbering system
CAS number:543-80-6
MDL number:MFCD00012447
EINECS number:208-849-0
RTECS number:AF4550000
BRN number:3693411
PubChem number:24855494
Physical property data
1. Properties: Colorless or white crystalline powder. Loses moisture at 110°C.
2. Density (g/mL, 25/4℃): 2.47
3. Relative vapor density (g/mL, air=1): Undetermined
4. Melting point (ºC): Undetermined
5. Boiling point (ºC, normal pressure): Undetermined
6. Boiling point (ºC, 5.2kPa): Undetermined
7. Refractive index: Undetermined
8. Flash point (ºC): Undetermined
9. Specific rotation (º): Undetermined
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (kPa, 25ºC): Undetermined
12. Saturated vapor Pressure (kPa, 60ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) partition coefficient: Undetermined
17. Explosion upper limit (%, V/V): Undetermined
18. Lower explosion limit (%, V/V): Undetermined
19. Solubility: soluble in water, slightly soluble in ethanol, 1g The product is dissolved in 1.5ml water and 700ml ethanol, and the aqueous solution has a neutral or slightly acidic reaction to litmus.
Toxicological data
1. Acute toxicity: Rat oral LD50: 921mg/kg, no detailed description except the lethal dose;
Mouse intravenous LC50: 21mg/kg, no detailed description except the lethal dose ;
Rabbit oral LDLo: 236 mg/kg, no detailed description except the lethal dose;
Rabbit subcutaneous LDLo: 96 mg/kg, no detailed description except the lethal dose;
Rabbit intravenous LDLo: 12mg/kg, no details except lethal dose;
Ecological data
This substance may be harmful to the environment, and special attention should be paid to water bodies.
Molecular structure data
None yet
Compute chemical data
1. Hydrophobic parameter calculation reference value (XlogP):
2.� Number of hydrogen bond donors: 0
3, Number of hydrogen bond acceptors: 4
4, Number of rotatable chemical bonds: 0
5, Interconversion Number of isomers:
6. Topological molecular polar surface area (TPSA): 80.3
7. Number of heavy atoms: 9
8. Surface charge: 0
9. Complexity: 25.5
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. The number of uncertain atomic stereocenters: 0
13. The number of determined chemical bond stereocenters: 0
14. The number of uncertain chemical bond stereocenters: 0
p>
15. Number of covalent bond units: 3
Properties and stability
1. Use and store according to specifications. It will not decompose and avoid contact with oxides.
2. Easily soluble in water and insoluble in ethanol. When barium acetate is recrystallized with water, the precipitated crystals are trihydrate when the temperature is below 24.7°C. The precipitated crystals are monohydrate at 24.7~41℃. The crystals precipitated above 41℃ are anhydrous. At 0°C, 100g of water can dissolve 59g of barium acetate. At 24.1℃, 78.1g of barium acetate can be dissolved in 100g of water. At 100℃, 75g of barium acetate can be dissolved in 100g of water. Used as a catalyst for organic reactions such as olefin polymerization.
Storage method
This product should be kept sealed.
Synthesis method
1. When barium acetate monohydrate is recrystallized with water, the crystals precipitated when the temperature is kept above 41°C are anhydrous barium acetate.
2. Anhydrous barium acetate can also be obtained by heating barium acetate monohydrate at 170°C for 12 hours while isolating carbon dioxide gas.
3.Prepared from the reaction of barium sulfide or barium hydroxide and acetic acid.
Purpose
1. Analysis of calcium. Precipitating agent for sulfates and chromates.
2. Catalyst for organic reactions. Mordant. lubricating oil.
3.Used as analytical reagents, such as precipitants and organic reaction catalysts.
