Benzanthracene
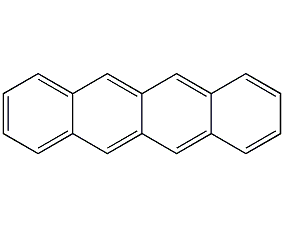

Structural formula
| Business number | 024G |
|---|---|
| Molecular formula | C18H12 |
| Molecular weight | 228.29 |
| label |
Tetracene, 2,3-benzanthracene, benzonaphthalene, 2,3-Benzanthracene, Benz[b]anthracene, Tetracene, Chrysogen, Aromatic hydrocarbons |
Numbering system
CAS number:92-24-0
MDL number:MFCD00003702
EINECS number:202-138-9
RTECS number:QI7605000
BRN number:1909299
PubChem number:24869382
Physical property data
1. Characteristics: Orange-red leaf-like crystals. There is slight green fluorescence under sunlight.
2. density (g/ml, 25/4 ℃): 1.35
3. Relative density (D 204) : 1.35
4. 5. boiling point (ºC, normal pressure): 437.6
6. Gas phase standard burning (焓) (kj · mol –1 </sub ): -9098.5
7. Gas phase standard claims heat (enthalpy) (kJ·mol-1): 291.4
8. The standard heating of the liquid phase standard (焓) (kj · mol -1 ): -7302.61
9. Liquid phase standard claimed heat (enthalpy) (kJ·mol-1): 78.50
10. Liquid phase standard entropy (J·mol-1·K-1): 214.18
11. Liquid phase standard formation free energy (kJ·mol-1): 217.8
12. Gas phase standard combustion heat (enthalpy) (kJ·mol-1): -9091.2
13. The gas phase standard claims heat (enthalpy) (kJ·mol-1): 293.0
14 . Standard standard heat of combustion (enthalpy) of crystalline phase (kJ·mol-1): -8969.0
15. Claimed heat (enthalpy) (kJ·mol-1): 170.8
16. The logarithmic value of the oil-water (octanol/water) partition coefficient: Undetermined
17. Explosion upper limit (%, V/V): Undetermined
18. Under explosionLimit (%, V/V): Undetermined
19. Solubility: Insoluble in most solvents, soluble in xylene, concentrated sulfuric acid, insoluble in benzene.
Toxicological data
None yet
Ecological data
None yet
Molecular structure data
1. Molar refractive index: 79.78
2. Molar volume (cm3/mol): 191.7
3. Isotonic specific volume (90.2K ): 518.7
4. Surface tension (dyne/cm): 53.5
5. Polarizability (10-24cm3): 31.62
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 0
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: None
6. Topological molecule polar surface area 0
7. Number of heavy atoms: 18
8. Surface charge: 0
9. Complexity: 236
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Exist in smoke.
2. Sublimation in vacuum.
3. Exists in coal tar.
Storage method
This product should be sealed and stored in a cool, dry place.
Synthesis method
1. Oxidize with chromic acid to obtain tetracene-5,12-quinone. Use 6-(2-carboxybenzyl)-1,2,3,4-tetrahydronaphthalene to treat it with zinc chloride and sodium chloride, and then dehydrogenate it.
2. It is formed by the condensation of succinic acid and phthalic anhydride in the presence of sodium acetate.
Purpose
Organic Synthesis.
